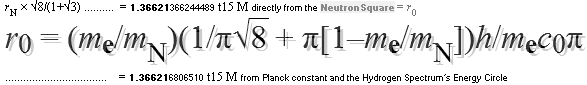THE
ATOMIC NUCLEUS II 2023IX10 | a ![]() production ·
| Senast uppdaterade version:
2025-03-16 · Universums Historia ♦ HumanRight is a knowledge domain
production ·
| Senast uppdaterade version:
2025-03-16 · Universums Historia ♦ HumanRight is a knowledge domain
content — innehåll
denna sida · webbSÖK äMNESORD på
denna sida Ctrl+F · sök ämnesord överallt i indexREGISTER · förteckning över all webbsites
PO4 ¦
ATOMIC MASS EQUATION — mATOM = Uu ¦
ATOMIC WEIGHT — U [ »relative atomic mass» ] ¦
ATOMIC MASS UNIT — u [ Dalton] = m[ 6C12 ]/12 ¦
ATOMIC MASS defect
EQUATION ¦
ComparingNUCLEAR
¦NScredit
Atomic masses — AtomicNucleus
— INTRODUCTION, nuclear radii, basic nuclides, comparing
early Weizsäcker solutions ¦ STANDARD UNIVERSAL
— divergence c0 = 2.99792458 T8 M/S is preserved
independent of gravitation.
TheAtomicNucleus I Jun2023 — DeducingTHErZ ¦ TAN II Sep2023 — comparing nuclear physics ¦ TAN III Nov2023 — relating Earth crust isotopic compositions ¦ TAN IV Dec2023 — FusionLimitMass FULIMA
EXPERIMENTALconfirmations: Atomic masses
mDweiz ¦ NSsolutions ¦ CONOR ¦ NSUunit ¦ PO4
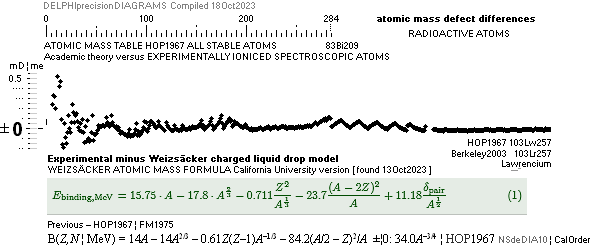
ATOMIC MASS, ATOMIC WEIGHT, ATOMIC MASS DEFECT, NUCLEAR MASS DEFECT, NUCLEAR BINDING ENERGY, AND THE ATOMIC MASS UNIT
——————————————————————————————————————————————————————————————————————
comparing fundamental
atomic-nuclear physics:
related-established
See Different Ways in comparing data between
theory and experimentally measured
Differences in
atomic mass defect electron masses — per mass number
MODERN ACADEMY IS OUTCLASSED BY TNED
— related physics and mathematics
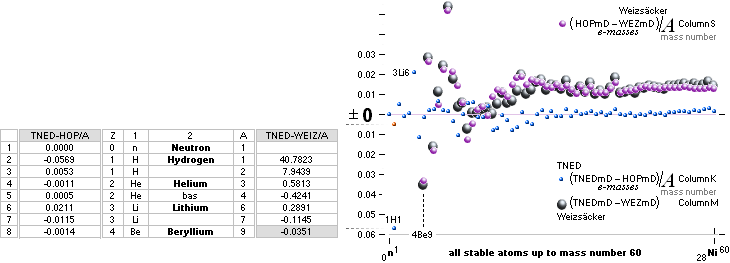
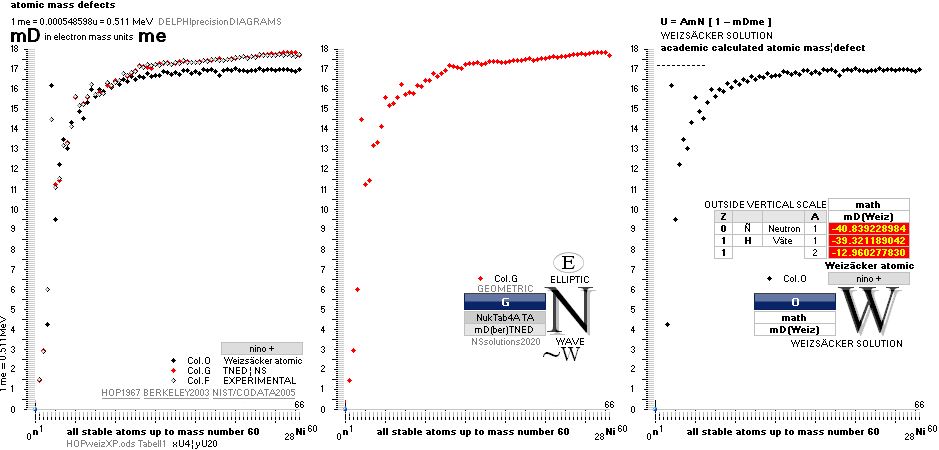
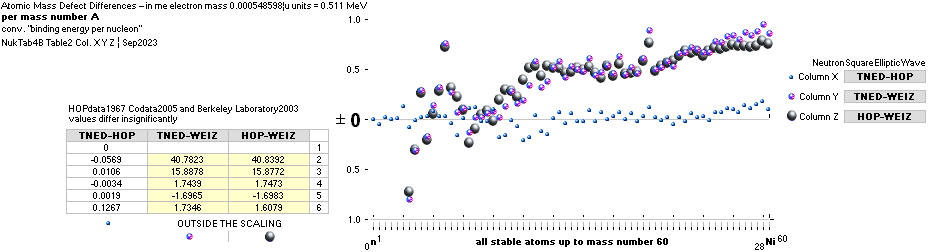
NuklidTab4A2023.ods Table4 — Columns KMS
Left table: The
7 left out ![]() Weizsäcker solution posts are far outside the
vertical scale. While the
Weizsäcker solution posts are far outside the
vertical scale. While the ![]() NS-solution have a more tight
experimental connection.
NS-solution have a more tight
experimental connection.
The diagram
above is of the same kind as the Comparing diagram. Only
difference: diagram above is Comp/A ( yielding ..
»per A-square» .. more tight Show).
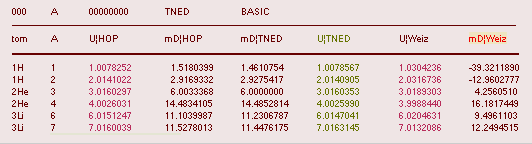
HOP¦WEZ mD = (
1 — [HOP¦WEZ U]/AmN)/me ¦ me
= 0.000548598¦u, mN = 1.0086652¦u, HOP¦WEIZSÄCKER U = element’s atomic mass in Dalton units u = 1.66033
t27 KG, A = mass number
HOPweizXP.ods Table1 ¦
NuklidTab4A2023.ods
Table4 — HOPtable1967, Weizsäcker
values¦ WEIZSÄCKER
EQUATION DETAILS ¦
ComparingTable
¦ WeizsäckerCALC
![]()
1H1-details: IN
GENERAL: THE NEUTRON SQUARE
SOLUTIONS OFFERER SEVERAL 3 WAYS TO CALCULATE
ATOMIC MASSES THROUGH ATOMIC MASS DEFECTS mD. This is an
example:
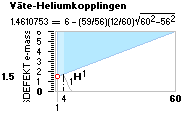
![]() mD ¦ NeutronSquare
= 6 — (59/56)(1/5)√ 60² — 56² = 1.461075377,
HOPmD = 1.518021871, (TNED–HOP)/(A=1) = -0.056946494
mD ¦ NeutronSquare
= 6 — (59/56)(1/5)√ 60² — 56² = 1.461075377,
HOPmD = 1.518021871, (TNED–HOP)/(A=1) = -0.056946494 ![]() basic
basic
![]() mD ¦ NeutronSquare = mD
mD ¦ NeutronSquare = mD![]() × (ProtonRadius/NeutronRadius
= [√8]/[1+√3]) = 1.512616535, (TNED–HOP)/(A=1) = -0.005405335
× (ProtonRadius/NeutronRadius
= [√8]/[1+√3]) = 1.512616535, (TNED–HOP)/(A=1) = -0.005405335 ![]() precision
precision
NOTE THAT EXPERIMENTAL VALUES USE SPECTROSCOPIC IONIZATION
TECHNIQUES, [WHERE THE ATOMS ARE ACCELERATED] AND HENCE reflect SLIGHT
DIFFERENT MASSES.
No explicit specific data on these spectroscopic ionization
[»mass affection»] data differences are known here.
The large 7
first Weizsäcker differences.
The Element isotopes from 1H1 to 3Li7 are left out in the diagram above as the
Weizsäcker values anyway lie far beyond the vertical scale. TNED
says — very close to experimentally measured values — the reason is because the
idea behind the Weizsäcker solutions — charged liquid drop mathematics — have
no corresponding atomic nuclear connection. Only when the atom grows larger and
heavier, that difference decreases. See further definite proofs [Jun2023] in THE ATOMIC
NUCLEUS.
EXCON: ExpCon
So much credit in present scientific literature is given to Albert Einstein (1905) on the formula E = mc2 — when in fact it is just a ride on (Max Planck 1900) the Planck constant h=mcr: E = hf = mcr/t = mc2. But the form also shows a (differentials ¦ x = yz : x/∞ = x/∞n = dx = dy · dz) more simple deducible nature (not mentioned: divergence energy):
dE = dF·dd = dma·dd = dm(c/t)dd = (m/∞)(c/t)(d/∞) = (m/∞)(c·d/t)(1/∞) = m(c2)(1/∞2) = m(c2)(1/∞) = dmc2: E = mc2.
dE = d(Fd) = d(mad) = d[m(c/t)d] = d[m(c·d/t)] = d(mc2); E = mc2. Or just: E=Fd=mad=m(c/t)d=mc2. No big deal.
• light does not connect kinetics Solar eclipse expeditions 1919+ — experimental confirmations:
c and v are not additive in physics — the vic-error — Michelson and Morley experiments 1881+
• Curved light paths does not develop centrifugal — kinetics — properties:
• light is massless — light is not gravitation — gravitation is not light — gravitation is NOT a particle:
• Max Planck was right, Albert Einstein was wrong:
The photoelectric effect reflects properties of the atoms (THE PERIODIC SYSTEM — resonance matrices) — not properties of light. Light propagates massless through electromagnetic Planck E=hf quanta named photons — also (inductively COEI conservation of energy by induction) interacting (Compton effect) with (±e nuclear structured) matter (atomic particles) where the Planck energy E=hf=mc2 always is conserved (ENERGY LAW ¦ POM):
EnergyLaw: energy cannot be created, nor destroyed, only converted. (±e nuclear structured) mass can be destroyed (COEI) because it cannot be created: light is massless. Particle proof that energy cannot create mass.
While the scientific community has the idea in particle physics, that energy and mass are substantial equivalents, related physics (Planck equivalents) explains mass-energy exchange properties: the Planck energy E=hf=mc2 is always conserved, under all circumstances. The scientific community introduced the idea of a spin property on the Einstein’s proposed massive photon — ”to make the chart complete”. In related physics, no such invention exist: the sum of all spins and moments in the atom is zero. When (Star physics) mass is destroyed E = (m→γ)c2, COEI certifies that the Planck energy E=hf=mc2 always is conserved.
The difference is excited (±e nuclear structured) gravitational mass, while modern corridors claim real steel gravitational mass creation — although also these aces know that mass cannot be created out of no mass.
The atomic nucleus compresses all these aspects in also verifying that all collected (Angeli2004) world data merges with the TNED deduced atomic and nuclear properties [The (rZ)2/r complex] — as verified from the coherent RevisitedHofstadter1956 electron scattering experiments: it all exposes the general charge-density distribution characteristics of the atomic nuclei — as so TNED confirmed.
— And so we can return to The Neutron Square — its Planck constant accounted confirmed deduced nuclear size properties — and their following connection to atomic mass properties for comparison on experimentally measured.
— Shorter: »we are just
warming up».
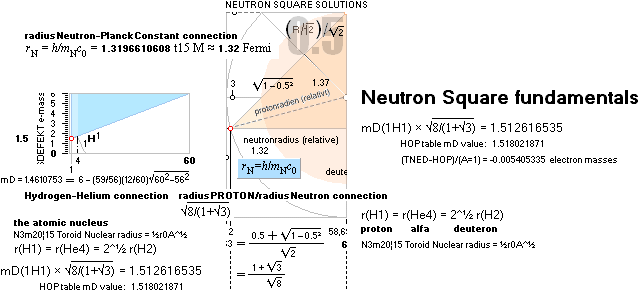
NeutronSquareFundamentals:
THE NEUTRAL ATOM — PROVISIONS
CosmicMATHrevelation ¦ Geometric
NeutronSquareSOLUTIONS ¦ VerticalELLIPTIC
For ToroRADIUS,
see the Deduction. Basics from ProtonRADIUS.
Neutron Square
atomic nuclear solutions has no physical or experimental foundational reference
at all, not a single point in space —
except
beginning from the Planck constant h = mcr: The Planck Ring:
the fundamental atom: the Neutron
(0°K, c=c0=2.99792458 T8
M/S: c0 is preserved as a natural constant independent of gravitation’s
influence DGD on local divergence = local
light propagation).
All mD(atomic
mass defect)-values emanate out of the Neutron Square and its deduced,
related and fully explained elliptic-trigonometric (wave)-hyperbolic
(CompCALu2023) mathematics equations — with so
basic geometric solutions to the first basic light atomic masses on their
atomic mass defects, mD. See FIBAPO Comparing Table.
NOTE: (ErrorTransferElectronMass) The 1967 HOP-table uncertainty value for 1H1 in mD electron masses is
specified as 0.02277 me (0.08 µu NuklidTab4B2023.ods TableA 4G) — see graph and diagram in ExperimentalErrors. The Neutron square 1H1 mD value difference on 0.0054 to the HOP-table’s is more
than four times less (and the HOP error and NS-difference cannot readily be compared). If appropriate, that is an unprecedented precision in pinpointing an
experimental result.
charge does not
connect mass — Never18
¦ NSsolutions
THE PIN POINTING QUESTION — no
yet found academic answer:
— In what way is the Measured Object’s Electric Charge involved in the object’s Measured mass?
Because the
Neutron Square Solution has
♦ no explicit nuclear charge (Z) connectivity
to atomic mass:
mD = (1 — U/AmN)/me —
no Z.
THE
ATOMIC NUCLEUS
¦ CompareQm
¦ Discovery
♦ nuclear charge does not connect atomic mass, nor nuclear size (rZ)²/r. No way.
ALTHOUGH HOWEVER THESE [±e nuclear structure] ARE INTEGRATED IN
THE ATOM
♦ charge DIVERGENCE — electric field — does not connect mass CONVERGENCE Physics756
EXPERIMENTAL nuclear-atomic PHYSICS [±e nuclear structure] CANNOT SEPARATE
THESE: BOTH NEEDED.
Modern academy educated freely available papers seem
not to get to the chase on relating the quest .. recycling .. NS
.. in the beginning ..
TNED — concentric atomic
production
Related physics and mathematics:
———————————————
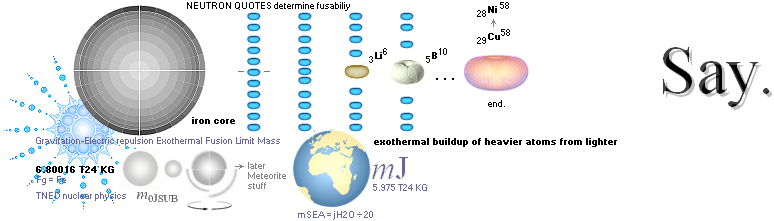
NUCLEAR FRAGMENTATION — How The K-cell Dmax is regained ¦ Exothermal nuclear reaction law ¦ NUCLEAR REACTION LAW ¦ GeneralCosmicStateLaw ¦ FusionRINGS
Odd and Even Nuclide Groups — why the core fusion body ends on IRON ¦ CWON from CAP ¦ The IRON CORE ¦ MilkyWay
SolarSystems
REGAINING — from the deduction of the exothermal nuclear reaction/fusion law
————————————————————————————————————
According to K1+K2–(m→γ)=K the Nuclear Reaction Law — related physics deduction — each composed atom and its nucleus from the point (light propagation c in space; see Comparing mathematics between related physics, Schwarzchild, and Einstein) and moment where the enveloping dominant gravitation has reduced divergence to c=0, a reversed situation is born. K+(m→γ=0)=K1+K2. If the atom and its nucleus finds exactly the g-mass corresponding to the energy emission (atomic mass defect) when that atom was formed, K apparently decays back to K1+K2. Further increasing gravitation pulls the net resulting fundamentally rebuilt Neutron (Planck constant h=mcr) masses into a state of Dmax until (COLLECTIVE bounce recoil when contacting on a max gravitation) a following Detonation occurs (The Incompressibility of the atomic nucleus). So it is all governed by gravitation. See further details from CosmoBasics (gravitational redshift, K-cell mathematics) unless already familiar (details in how the expansion and contraction works by related mathematics and physics — modern academic confusion »dark energy» on motion and general cosmological gravitational redshift, no motion: no »dark energy»).
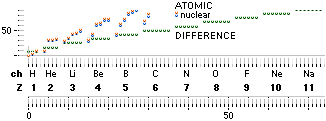
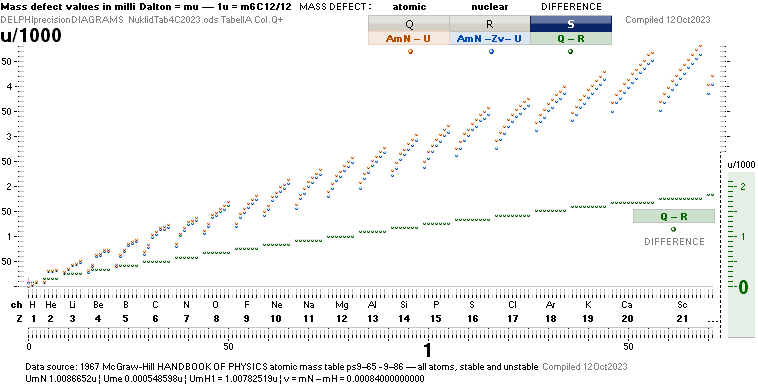
ComAtNu: Comparing Atomic and Nuclear mass
defect concepts and values — clarifying the terminology
Comparing Atomic and Nuclear
CLARIFYING THE TERMINOLOGY — by experimentally measured examples
Explaining ¦ EXAMPLIFYING
THE DIFFERENCE BETWEEN ATOMIC AND NUCLEAR MASS DEFECT
ATOMIC AmN – U
the work mass-energy WASTE needed to build the
atom [Sw., arbetets massa-till-
energi bortfall: gravitell svinn-massa; g-mass-svinn].
NUCLEAR AmN – U – Zv ¦ v = mN – m1H1 = 0.00084u ¦ U = mATOM/u ¦ u = m(6C12)/12 = 1 Dalton
mN = neutron mass 1.0086652u,
A
= mass number = number of FAMQ fundamental atomic mass Quanta mN
that built the atom ¦ u = 1.66033 t27 KG ¦ Z = atomic number = the atom’s nuclear
electric +charge, same as
the atom’s electron mass electric -charge.
the work mass-energy WASTE needed to build the
atom — minus the Zv mass energy work waste:
v is the NEUTRONIC n nuclear structural
mass energy WASTE equivalent 0.00084u in
becoming [electric displacement rearranging work] the PROTONIC p nuclear structural
property
integrating the fundamental PROTONIC p nuclear
structure with the fundamental NEUTRONIC n nuclear structure into the
fundamental atomic NUCLEAR — in related physics NOT the
academic nucleonic — structure.
While the academic idea is that
of »separately inside the nucleus
spinning neutrons and protons», related physics is dealing with a fundamental nuclear
electric displacement ±e structure (Planck fractal structural ring
constant): no inside spinning particles — as verified in the (rZ)²/r complex (The
Atomic Nucleus).
Weizsäcker nuclear binding energy form: (AmN – U – Zv)
nuclear mass defect.
Weizsäcker atomic binding energy form: (AmN – U)
atomic mass defect; Weiz + Zv.
Related physics — particle and
unity
AS IT IS WITH MERGING WATERS
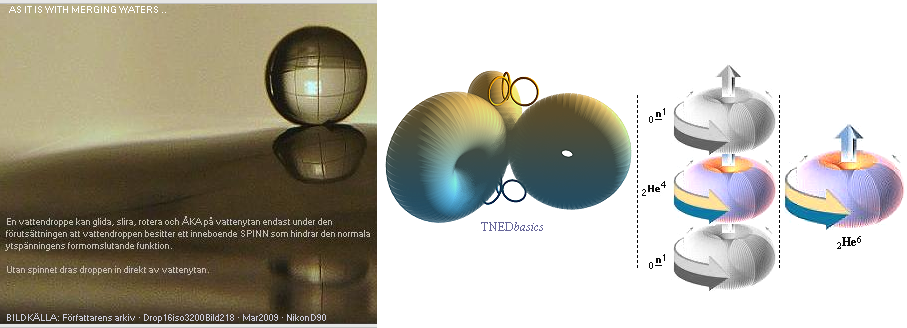
———————————————
CREATION
OR DIVISION — nuclear fragments¦ SPIN
SYNCHRONIZATION ¦ The 3Li8 Nuclide ¦ The Water
Surface Spinning GIF water drop ¦ UNIT WITHOUT PARTS — nature illustrates the
principles
EXCON details: While the scientific community (1905+) has adopted the idea in particle physics, that energy and mass are substantial equivalents — real physical substantial gravitational particle mass creation — related physics (Planck equivalents) explains the Planck constant h=mcr energy-mass equivalence E=hf=mcr/t=mc2 on a principle of exchange properties: the Planck energy E=hf=mc2 is always conserved, under all circumstances. Meaning: When (±e nuclear structured) matter (atomic particles) merge from lighter to heavier atoms/elements — where the Planck energy E=hf=mc2 always is conserved — the general RULE in mathematical physics becomes that the reverse also holds — however with a resulting excited fission product (atomic and nuclear mass defect concepts). In modern quarters, this energy split product is — with no exception — always implied as a fully substantial gravitational spouse. In related physics that is a grave delusion, and a most honest deep and real steel fundamental physical impossibility;
ENERGY LAW ¦ POM ¦ EnergyLaw: energy cannot be created, nor destroyed, only converted. (±e nuclear structured) mass can be destroyed (inductively COEI conservation of energy by induction) because it (substantial gravitational ±e nuclear structured) cannot be created: light is massless — Particle proof that energy cannot create mass. When (Star physics) mass is destroyed E = (m→γ)c2, COEI certifies that the Planck energy E=hf=mc2 always is conserved: light and heat.
Max Planck was right. Albert Einstein was wrong. No doubt (EXCON): light is massless. No gravitation. No developed centrifugal property in curved light paths. No way.
— See more comparing academic related details in EXPERIMENTAL CONFIRMATIONS, unless already familiar.
The most interesting aspect (not explained):
— The experimental measure of the neutron mass:
— Its precision.
It cannot be directly measured (yet).
We should not be overly
enthusiastic on raising the experimentally measured atomic masses values to the
heavens, without certifying that the influence of the experimental ”mass-to
charge” complex not significantly affects the actual neutral
atomic mass. How do we know?
neutral to
experimental
We can put it this way — testing how the NS solutions conform on the true physical ones:
— HOW DO WE GET RID OF THE EXPERIMENTAL CHARGE electric
magnetic FROM THE EXPERIMENTAL RESULTS — in REFINING THE WHOLE PICTURE TO
INCLUDE ONLY THE NET mass VALUES?
The neutral atom. The NS
— for comparing purposes.
— We can’t (says the present scientific PhD community as one man, do correct) — unless we have a Gauge — a preference Index — by which to perform explicit advanced experimental tests:
— Telling us what to look for. At present, no other is known here than The NS solutions.
” Because atoms are much to small to measure individually and do not have a charge, there is no convenient way to accurately measure absolute atomic masses.”,
” Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer.”,
” The technique is conceptually similar to the one Thomson used to determine the mass-to-charge ratio of the electron.”,
” First, electrons are removed from or added to atoms or molecules, thus producing charged particles called ions. When an electric field is applied, the ions are accelerated into a separate chamber where they are deflected from their initial trajectory by a magnetic field, like the electrons in Thomson’s experiment.”,
” The extent of the deflection depends on the mass-to-charge ratio of the ion.”,
CHAPTER
1.6: ISOTOPES AND ATOMIC MASSES, Howard University
Libre texts — Chemistry — No
date reference ( .. perhaps 1634 .. they were psychic .. ),
sampled @Internet 7Sep2023
THE INTRODUCED ELECTRIC CHARGE ADDS EXTRA MASS SPECTROSCOPIC DEFLECTING POWER
related physics: mass and charge — massless electric field: light c property — does not connect
What we know: the experimental
charge dependency cannot be separated from the actual mass spectroscopic
experiment — by no other means than through a (thorough) theoretical insight:
some real steel knowledge of the actual mechanism. To trap it, also
experimentally, a first encouraging insight must exist that IT is missing.
What we do know is that nuclear
charge (Z) and nuclear mass (m) frequently is used in established works
on nuclear (rms) charge radii determinations: nuclear mass and
nuclear charge are implied physical cooperators — while (rZ)²/r related physics
(Angeli2004data) proves that the charge dependent idea as such to be more of an
experimental necessity than a true real physical property: nuclear charge has
no volumetric physical reality: Z is a nuclear surface structure extension (NuclearStructure),
and has no connectivity to nuclear size — other than the fact that electric
charge cannot be removed from atomic/nuclear experimental investigation.
—»It is included in the
general computer modeling mathematics».
— It is taken for granted —
but has no physical correspondence — related physics TNED NeutronSquare
solutions says.
So: how is it?
The tight Neutron Square solutions NSdeDIA1 compared to the experimental values
strongly suggests — still here with no other proofs — that there really is »an
experimental charge issue» in the complex of experimentally measuring atomic
masses. Namely also so: Outside the NS solutions (unknown in modern corridors) nothing reflects even such a
possibility: it lies hidden, dormant. The experimental charge »issue» becomes
apparent first when the NS complex becomes uncovered: mass and charge does not
interact, although intrinsically integrated: the atom, the fundamental form of
gravitation.
Continue on
THE UNIVERSAL NATURE OF THE
NEUTRON SQUARE — how reliable
is it?
and
CosmicMATHrevelation — proving the real steel essence of nature ..
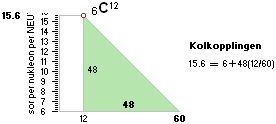
NS8Oct2023: NSUunit: Provisions¦ NScredit ¦ CAUSE ¦ Normalization
TheQuest of
THE ATOMIC MASS UNIT u=m(6C12)/12
PREFERENCE
— IN THE NEUTRON SQUARE mD
Exacting The A=12
IS THE NEUTRON SQUARE SOLUTIONS COMPLEX VALID EVERYWHERE,
UNDER ALL POSSIBLE CONDITIONS, NO EXCEPTIONS?
QUESTIONING A UNIVERSAL NATURE of THE NS
NEUTRON SQUARE SOLUTIONS
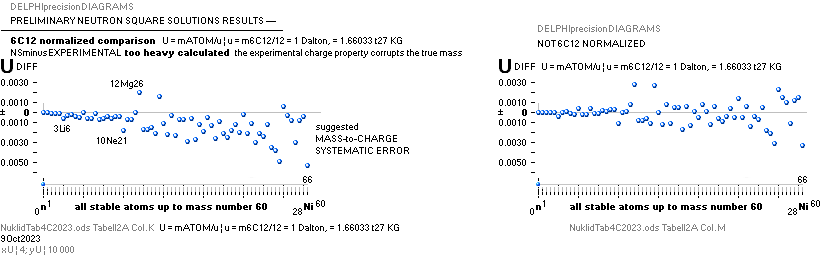
NuklidTab4C2023.ods Tabell 2A Col.M ¦ TheActualDifferences
The basic apparently highly experimentally matching NEUTRAL ATOMIC NEUTRON SQUARE SOLUTIONS NS solutions ¦ TheActualDifferences has introduced a QUESTIONING
central atomic mass defect equation
mD = (1 – U/AmN)/me. THROUGH
ITS U FORM U = m/u ¦ m = Uu
U = AmN(1 – mDme) ¦
THE neutral atom’s atomic weight U = m/u CAN BE SPECIFIED FROM A UNIVERSAL
u = m(6C12)/12 = 1 Dalton = 1 cosmological atomic mass
unit. The proof below. Original Swedish deduction 2008.
Universal atomic
mass unit
Giving any a universal credit to such a suggested atomic
cosmologically valid m(6C12)/12 mass unit u, its NS suggested atomic mass
defect (total
atomic binding energy)
mD value is exactly 15.6 electron masses. NS solutions
Proof (NScredit ¦ Discovery) :
Only WHERE — and IF — a single unique
m/u = U = A exists will
define a corresponding 1 = mN(1—mDme), mN = 1/(1—mDme); mN/me = 1/(me — mDme²). Only one, otherwise no solid
preference. mD = (1 — 1/mN)/me = Neutron
Square order geometrical mathematics = 6 + (60—A)(12/60) = 6 + 12 — 12A/60
= 18 — A/5 = 6 + 12(1 — A/60) — 12/60 = 1/5 is a scale
transfer
NuklidTab4C2023.ods Tabell1 A260+ —
exemplifying different me and mN (Wikipedia mNw mew), clarifying the above
validity test for a single U=A.
In modern quarters, no such explicit occurrence is known (CU). And the academic choice of m6C12/12 (1961 ¦ 1993 coined: one Dalton ¦ 2005 IUPAP) therefore appears arbitrary on »a
best fit» (preferred in chemistry) basis (earlier
8O16, first 1H1).
Normalization — see end comparing results in CONOR
The u-condition — U = A only one — not several, under no
conditions ever — single unique occasion: it defines the proof:
mN = 1/(1 – mDme)
WE ONLY HAVE TO FIND ONE ANY THE FIRST mD SOLUTION — ANY. Just a first. Any — if at all:
GEOMETRIC ¦
NuklidTab4C2023.ods Tabell1 A260+
The mNw
and mew are the present (Oct2023) Wikipedia reported values for neutron
and electron mass in Dalton units.
In calculating mD
=18–12A/60 all the mD:s for all the mass numbers A, then
calculating/seeking U = AmN(1–mDme) a corresponding
integer U=A will always return the same answer 12 — provided (CAUSE) the used mN
amd me follow the Neutron square suggested general solution’s u-equation
provision
mN = 1/(1 — 15.6me) OR me = (1 — 1/mN)15.6. The precision of the me
or mN is insignificant;
— A test column
(above
right) with an me=0.5 giving an mN=-0.1470588235
still returns a single U=A=12, all other have decimal tails. 6C12 it is. A17,
17Clorine35.
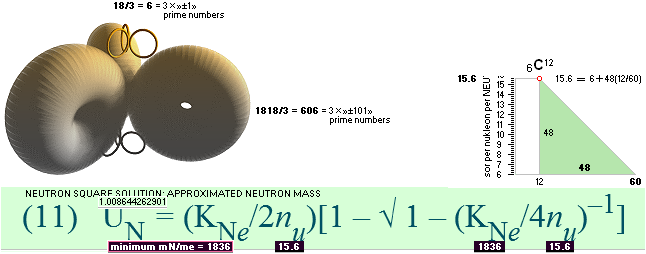
The approximated solution: mN/me = 1836minimum; U = (1836/2[15.6])(1 — √ 1 — 4(15.6)/1836) = mN = 1.008644262901; me = mN/1836 = 0.0005493705; mD = 15.6 = 18 — A/5 ; A = 5(18 — 15.6) = 12.
FROM THIS RESULT, IT DOES NO LONGER MATTER WHICH SPECIFIC mD or me — institution, laboratory, region, epoch — WE USE, BECAUSE THE UNIQUE
u-CONNECTION mN = 1/(1 — 15.6me) OR me = (1 — 1/mN)15.6 WILL AUTOMATICALLY ALWAYS CORRECT ON THE SAME END STATION RESULT: m(6C12)/12=u.
BUT THIS mD15.6me NS PREFERENCE ALSO
BREACHES ON THE ESTABLISHED EXPERIMENTAL mN¦me PROVISIONS. Type
HOPmN 1.0086652u from the HOPme 0.000548598u: 1/(1 – 15.6me) = 1.0086320026u IS NOT THE SAME AS the HOPmN 1.0086652u. NS SUGGESTING: The experimentally measured masses involves electric and magnetic — not neutral — provisions, and have — therefore — no exact corresponding experimentally proven neutral quantities matching the Neutron Square guaranteed neutral atomic mass defect (atomic binding energy) suggested values.
WHEN IT COMES TO THE QUOTE mN/me THESE DIFFERENCES ARE MINOR (not directly significant);
HOP mN/me = 1838.6235458387. 1818 + 18 + 2.624.
HOP mN’/me = 1838.5630326577. 1818 + 18 + 2.563. There is a 0.00329% deviation in the relation. We know not here how plausible such is experimentally in measuring mN.
THESE RESULTS MIGHT ALERT A CHALLENGE. NAMELY IN
COMPARING THE EXPERIMENTALLY MEASURED ATOMIC MASSES/WEIGHTS WITH THE
CORRESPONDING NEUTRON SQUARE VALUES, A NORMALIZATION MUST BE MADE ON THE
EXPERIMENTAL [mN’ = 1/(1 – 15.6me)] TO SECURE THE NS REFERENCE ON
THE UNIVERSAL ATOMIC MASS UNIT m(6C12)/12=u AS STATED.
Normally — no adjustments — we just would translate the experimental
U values to NS mD values by the given
Uexp → mD = (1 — Uexp/AmN)/me — given the laboratory or institutional
mN and me values.
However in sequring the NS named m(6C12)/12=u
transfer, the translation must respect an atomic mass unit normalization
Uexp → mD = (1 — Uexp/AmN’)/me, = (1 — Uexp/A[1/(1—15.6me)])/me, = (1 — (1—15.6me)Uexp/A)/me.
THE EXPERIMENTAL U IS FORCED MODIFIED THEN. AND THERE IS
NOTHING WE CAN DO ABOUT THAT IF AN EXACT NS ATOMIC MASS UNIT REFERENCE
COMPARISON IS ON THE TABLE. If it isn’t, the NS values have no true and
fair comparing meaning, and only the experimentally values count.
That is an
especially sensitive task when comparing on the new (WeizCalifornia)
Weizsäcker solution quantities — which we already know is aiming at a
persistent academic chase on the (new popular academic computer modeling)
experimental (NScredit). The NS solution values will
breach that dependence: The Weizsäcker quantities (uniformly charged liquid drop nucleonic
model) have
absolutely no connection to the (TNED toroid Planck constant) NS complex.
No way. The comparing diagrams will underline that position (CONOR
¦ ComNorm).
QUESTIONING A UNIVERSAL NATURE of
THE NS NEUTRON
SQUARE SOLUTIONS
———————————————
Absolute
metrics ¦ The Absolut METRIC ¦ The
ATOMIC NUCLEUS ¦ NEUTRON SQUARE SOLUTIONS
¦ NSdeDIA1 ¦ Geometric
—————————————————————————————————————————————————————————————
9Oct2023:
U = AmN(1 – mDme) ; AtomicMassDefectEquation
12 = 12X(1 – 15.6me) ;
1 = X(1 – 15.6me) ;
X = 1/(1 – 15.6me) ;
me = 0.000548598 u ; Wikipedia Oct2023: 0.000548579909065
X = 1.008632002589970 ; »NSexMatchTractor»:
=
mXN UNIVERSAL neutron square
Experimental Mass-to-Charge Transfer Corrector
—————————————————————————————————————————————————————————————
THE NEUTRON SQUARE SOLUTIONS EXPLANATION
Normalized comparison
NuklidTab4C2023.ods Tabell2A Col.K ¦ M
———————————————
THE DISCOVERY — TNED Universe History
The original
ideas (2003) leading to the NS
atomic mass defect (mD) chart:
♦ NuclearSTRUCTURE: (HOP values) mN/me = 1838.6235458387.
PRIME NUMBERS: 1836 + 2.6235458387 = 1818 + 18 + k (BASICmN):
1818 + 18
———————— = 606 + 6 = 3 × (»±101» + »±1»)
A prime number certifies a basic
barrier on whole number divisibility (Periodic system matrix basics — it is all about structure: resonances: basic whole
number solutions): ±e NuclearSTRUCTURE.
♦ Scrutinizing (2003) the 1967 HOP table on atomic
masses, comparing on a first drafted nuclide chart (TheoryExperiment)
immediately revealed a possible apparent elliptic (Paintbrush, Windows 3.1) morphological
complex — taken on (exothermal) fusion paths (Nuclide/FusionRING ¦ mDmethod
¦ Geometric
¦ VerticalElliptic).
♦ Attempting to deduce a connection on elliptic equations (EllipticEquation
¦ Deduction),
immediately gave response:
♦ The Neutron Square discovery (2003) was a fact (Comparing
Experimental/NS).
— Never mentioned in modern quarters.
On Aug2008 this UH UniverseHistory domain was introduced @Internet — on
the NS discovery credit alone (»a new model of the universe»).
———————————————
ProtonRADIUS ¦
NuclearSTRUCTURE
¦ Atomic Mass Defect — ATOMIC MASS UNIT — deducing the atomic
mass unit
See also — mass-to-charge dependency
issue:
The elementary mass-charge
independence (Planck equivalents deductions)
NOTE the related deduction to the electric charge (Q), not mentioned in modern quarters:
———————————————
No matter the electric and magnetic influence on the
electron mass (e) — independent of any type of experimentation — under all
conditions, no exceptions:
Q is preserved — unless ±e annihilated (COEI
conservation of energy by induction).
The proportionality between affected mass (m) over (space
electric) resistance (R) guarantees that electric (particle) acceleration (Planck equivalents
as deduced)
leaves the electric charge (Q) intact.
Assuming, or implying, any idea of ”mass-to-charge ratio”
without proper specification and definition, will no doubt expose hazard to the
reader.
As we know: Atomic Particle experimentation is dependent on charge
properties. But the academic (Wikipedia Windows) insight into these physical
domains seems less developed than the ambition to present modeling data on
their nature: the atomic nucleus.
See further in
•
the neutral and optimal unaffected conditions under which the Neutron
Square Solution NS neutral atomic mass defect (mD) values can be understood to apply.
— precision (signal) measuring under
significantly different gravitational conditions.
ClarifyingTheUequation:
17Oct2023
It belongs to a common
understanding that the energy needed to complete an assembly is the same energy
reversed to restore the original — ideally »the movie backwards».
See Wikipedia quote in Not
represented.
But the present common science set of terms
and concepts apparently exposes some lack of a developed and well related,
explainable and understandable vocabulary. The reader will be the judge on that
in the following.
aMAD: CTU
Necessary clarifying concepts
from established literature — related, compared and exemplified
ATOMIC MASS DEFECT AND NUCLEAR MASS DEFECT —
MATHEMATICAL EXPRESSIONS AND CONCEPTS
The number (A) of Fundamental Atom Quanta (FAMQ) — the neutron mass mN — that made the atom (a), minus the actually (experimentally) measured atomic mass U=mATOM/(u=m[6C12]/12=1Dalton), defines the total mass-energy waste Work MDa needed to complete (a), taken from the masses of AmN through the Planck mass energy equivalent E = hf = mcr/t = mc2.
atomic mass defect MDa = AmN – U ¦ U = mATOM/u ¦ u = m(6C12)/6 = 1 Dalton
U = AmN – MDa
nuclear mass defect MDn = AmN – U – Zv ¦ v = nino = mN – (mP + me) = mN – m1H1 (= 0.00084u)
MDn – Zv = MDa MDn = MDa + Zv.
v: the work mass energy waste that built the first atom — 1H1
Hydrogen — from the fundamental atom: The Neutron [ FAMQ ]
SEE ILLUSTRATED FROM EXPERIMENTAL VALUES IN Comparing nuclear AND AtomDiffNuc.
— As above: When there is
vocabulary lack of terms in an established corridor, still attempting to handle
the different domains, inevitable confusions will most certainly arise: we have
to find safe and clear examples in closing out such traps in the descriptions.
Compare Examples.
NUCELAR mass defect general expression: — WikiWEIZnuclearMass exemplifying by quote
E(MeV)/A = (AmN – U –
Zv)
× uc02/(A × T6 × e) nuclear
T6 = 106 — in UH we use the simplification Tt for 10^± ¦ E = UQ = mc2 ¦ U(eVOLT) = mc2/(Q=e) ¦ U(MEGAeVOLT) = mc2/(T6 e)
ATOMIC mass defect general expression:
E(MeV)/A = (AmN – U) × uc02/(A
× T6 × e)
atomic
COMPARING EQUALITIES — provided gravitational masses only — me electron mass
mNUC = U – Zme ¦ related: nucleus gravitating mass + electrons gravitating mass = atom gravitating mass; Rest: 0.
=
AmN – MDa
– Zme ; WikiWeizNuclearMASS
— On a RELATED basis — to Check and say:
There is no way
to express this equality OTHER
than INCLUDING
these factors; Rest: 0:
The mNUC nuclear mass comes first after the mATOM has been assembled. So, it relates to U, not to AmN;
= AmN – (MDn – Zv) – Zme
= AmN – MDn + Zv – Zme . No way.
When n neutrons decay, lying inside of each others nuclear barriers (the nuclear delimiting sphere), and so performs a fast phase of spontaneous fusions, the resulting atom’s binding energy can not bring these original neutrons back on their original masses. These were partly wasted by working mass-energy during the fusion phase, the actual atomic mass defect.
Compare: the Planck equivalent mass-energy EXCHANGE term in EXCON: related physics.
See also NUCLEAR REACTION LAW.
aMAD ¦ CTU ¦ UnequivocalAmD
CLARIFYING attempt on THE TERMINOLOGY AND ITS RELATED MEANING
MDa = AmN – U
———————————————————————————————
(1) U = AmN – mDme ¦ simplified —— when we know the meaning
of the parameters:
U = atomic mass m per atomic mass unit u = atomic weight — ”relative atomic
mass”
mN fundamental
atomic mass quantity QUANTUM
— the neutron, enveloping the Hydrogen Atom
A number
of mN that built the A atom
also called MASS NUMBER — ”number of protons Z plus neutrons n”
mD IN RELATED PHYSICS:
atomic mass defect —
the Planck energy E = hf = mcr/t = mc² working energy wasted
on building the A atom;
me electron
mass — 0.000548598 u
here in UH, = 0.511 MeV:
♦ atomic mass is the raw A neutron quanta
mass minus the energy building work
Related (1):
Left side of the (1) rank is the (U) atomic mass expressed in Dalton units (u).
Right side of the rank’s first term AmN is the Fundamental Atomic Mass Quantum (FAMQ) the neutron mass (mN = 1.0086652) times the atom’s mass number (A) from which the U atom was built. The second term is the per mass number (A) atomic mass defect mD (Planck work hf-energy E=mc² equivalent) in number of electron mass units, times the electron mass (me, 0.000548598) in Dalton units.
The complete left side U value of the whole atomic mass on the right side is
(2) U = AmN – mDme × AmN
completing the third term also for the whole atomic mass. Hence
(3) U = AmN(1 – mDme), = AmN – MDa .
There is no dependency on electric charge in this mutual equality equation.
Through the deduced Neutron Square general elliptic equation
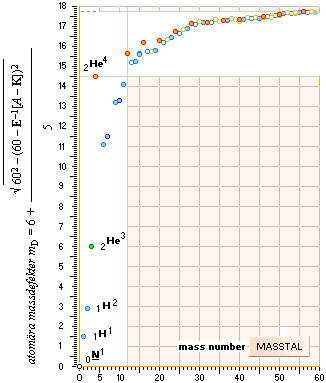
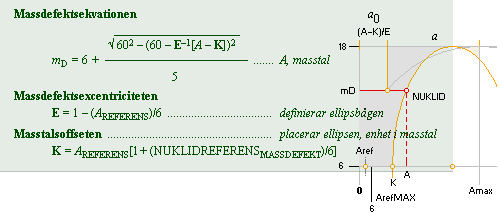
mD = 6 + (1/5)√ 60² — (60 — [ A–K]²)/E see NSsolutions
mD values are not entirely dependent on the mass number (A).
— The (EllipticEquation) KE factors connect FUSION PATHS (connecting elliptic arcs) defining (VerticalElliptic) the end atom — still no charge (Z) dependency.
Or so automatically integrated with no direct need for a separate analysis.
So (conclusion):
THE (UnequivocalAmD) ASSUMPTION IN NS SOLUTIONS THAT ISOBARIC ATOMS (same A, different U) IN PHYSICAL PRACTICE WOULD HAVE A MEAN AVERAGE ATOMIC MASS WILL NOT HOLD — except as a simplified violation on the true masses.
• Atoms with same mD can have different U from different A.
• Atoms with same mD can have different A.
• Atoms with same A can have different U from different mD.
— In NS solutions it is the mD Elliptic equation (KE) that secures also different U from same A, depending on how the end atom product becomes assembled on its A fusion production path. There is still no parametric electric (nuclear) charge dependency in these expressions.
See specific comparing isobars between experimental values and NS in
— there is a suggested (systematic) significant difference.
CosmicMATHrevelation: 25Sep2023 —
Never18 ¦ Geometric
Modern academy teaching system with its
merits and general moral and educative standards, a short review:
We
do NOT intend to be rude — just fair and just. Have your say. EXAMPLE: A
student who cannot »keep up» with the competition, commits suicide. Real world
situation. Unfortunately, a not very seldom happening inside modern quarters
educational institutions history. There are several examples — through several
disciplines.
—
The AIM of science and knowledge — HumanRight recognition standards; decency, respect, honour — was NOT to
kill. It was to teach and educate: to share. As life has evolved with cooperating
organs to a single admirable individual. The aim was NOT to profit, not to push or stress — individual
care. The natural aim was a respectful cooperation. HumanRight recognition. ”.. every individual and every organ
in society ..”. 24/7.
—
And how is it, what’s up? Not a word. Not a spell. Not a sound. Modern academic teaching system
cannot handle that quest. Most certainly not. It is — appears — a complete abstract to present
academic thinking. Not existent. No say.
—
2023: Humanity continues to be brought into the biological dark:
GUARANTEED
PRESERVED BIODIVERSITY —— on a never disturbed natural root fungi — TRDWS: biodiversity
suffocates with the kill of alive trees — THE WORLD DEMOCRACY CRISIS PHENOMENA. RespondingNature.
HEALTH.
” Människan har
skapat matematiken. Därom är alla lärde överens”,
Vetandets Värld 25Mar1988, Sveriges Radio.
English:
”Man has created
mathematics. Thereof are all the educated in agreement.”,
SWEDISH RADIO, the weekly Knowledge World radio program
25Mar1988
— The Swedish definition of Education: »you are not welcome here». Very high
IQ. MustBuyBook.
Delar Svenska Vetenskapsakademin ut någon annan behörighet
än den som innefattar kalhyggen, förstörd natur? Det ligger ingen medveten
illasinnad tanke bakom. Men det är vad ögat ser och hjärtat känner.
Naturfientligt utvecklad teknik. Var finns ett deklarerat
bestämt avståndstagande, en erinran om respekterad
människorätt?
”.. varje individ och varje organ i samhället ..”.
” matematik
Enligt etablerad uppfattning är matematiken läran om tal, om rummet, och
de många generaliseringar av dessa begrepp, som skapats av det mänskliga
intellejtet,”,
MATEMATIKLEXIKON W&W 1991 s278sp2ö
English:
”mathematics According to established ideas mathematics is the teaching
of numbers, of space, and the many generalizations of these concepts, having
been created by the human intellect.”,
Swedish version of THE
CRESCENT DICTIONARY OF MATHEMATICS (1962), W. Karush
— Test
»formulated» for »created». Then we can talk.
Summing:
By a natural DRIFT of ignorance, not deliberately or by any such
planning: Modern 1800+ academic ideas of our origin is holding mankind in a
mental prison.
— Disclaim that, and we will surrender immediately.
Mankind 1800+ became locked up by [ apparently in many ways
on Natural Destructive ] authoritative merits and ideas about moral and
educational standards. These still prevent the individual from developing a natural insight: not one word
HumanRight recognition. The below stuff is part of the proof:
modern academic thinking is intrinsically continuing
to present proofs that works against any idea of intelligence other
that itself.
Again: by Drift.
Not plan. Against nature. Not with her. It holds its own present established
academic teaching system to be a product of type »absolute intelligence» — on a
not seldom favorized foremost example (”nothing lasts forever”, popular
academic entropy study): Our universe was created in the past out of nothing,
and it will die when the stars run out of fuel, and there is nothing any one
can do about that. Cutting out every nerve and tissue out of the human child
mind. Very high IQ stuff. MustBuyBook. — ».. but if there is a deep meaning,
why don’t we see it?». TEST STOP DENYING IT.
COMPARE THE REAL STEEL WORLD OF NATURE, AS EXPERIENCED:
Man discovers mathematics:
The paragons from natural observations give us hints in how to develop a
formulating language. Man creates no math at all — except provably
faulty statements. The LIST.
Say.
— What was never
created, timeless, perfectly solid, no exception.
Shorter: perfect
flawless joy. Say.
— We are not here to weaken Defense. We
are here to make it stronger, deeper, more fundamental.
THE GUARD OF CIVILIZATION
CosmicMATHrevelation ¦ Resurrection11Sep2023 ¦ AtomicMassDefectEquation
Geometric: NeutronSquareSolutions ¦ VerticalELLIPTIC ¦ NeutronSquareFundamentals ¦
neutral to
experimental ——
U = mATOM/uC12÷12 — traditional Atomic Weight, present as Relative Atomic Mass,
u =
1.66033 t27 KG, 1 Dalton — beginning from the neutron Planck
ring h = mcr
CONCURRENT ATOMIC MASSES: U = AmN(1–mDme)
¦ mD = 6 ± k(1/5)√ 60²–(60–A)² ¦ Never18
Neutron Square Solutions
— atomic nuclear physics — as testified by experimental results — absolute
neutral atom preference — NEUTRAL ATOMIC Weights/MASSES

NuklidTab4B2023.ods Table1 Col.AI+ — The simple amazing astounding coherences that, apparently, nobody
can deny — never noticed in modern quarters. No way.
The 7 exemplified Neutron Square paragonic
parts below are tabled above in comparing experimentally measured results.
HOP, experimentally
measured, NS, NeutronSquare solutions — in this
UniverseHistory beginning from 2003+ on a Windows XP machine [with Windows 3.1
Paintbrush] and the HOP table data.
The
experimental coherence NSdeDIA1 is no doubt astounding (EXPERIMENTALconfirmations).
Compare modern
academic standard (Entropy Quote),
the
observed and noted1800+ central instance of denial of the cosmic nature: »nothing lasts forever» — the apparent modern academic 1800+invention
or more
fairly expressed, a modern academic dictated delusion to keep the timeless
truth of nature on a safe distance — with zero
physical solidity. Compare the rhetoric response: If you say so, that nothing lasts
forever, when does that cease? Wake up to reality.
These paragon
»matrix formations» apparently have no origin in time: time never began — realize
it, read it and weep. As the Pythagorean Theorem,
it can be forgotten but never destroyed — and rediscovered, any
amount of times — without changing the least or the smallest, not at all. The
atomic mass defect values from the geometric neutron square solutions have no
physical or experimental foundation — except the Planck constant: the neutron,
the fundamental atom: h=mcr. Testing the experimentally found values on
the basic Planck ring principle, made the neutron square solutions appear
immediately — concurrent in values (2003 in this history).
— There is
apparently no way to undo these discovered results — never known, and never
asked for, in modern corridors. Shorter:
— Why was the
above never presented from the academic aces — What’sUp — why is it unknown in
there?
— Educate us.
Modern academic cosmological idea is so occupied by
its own supreme standard, that it has forgotten how it came that it locked
humanity into a dark box.
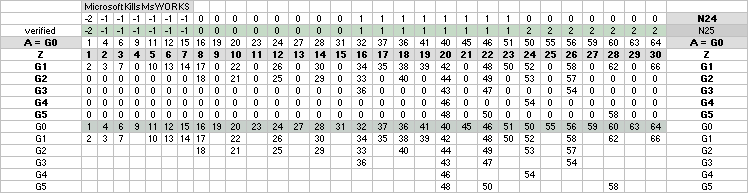
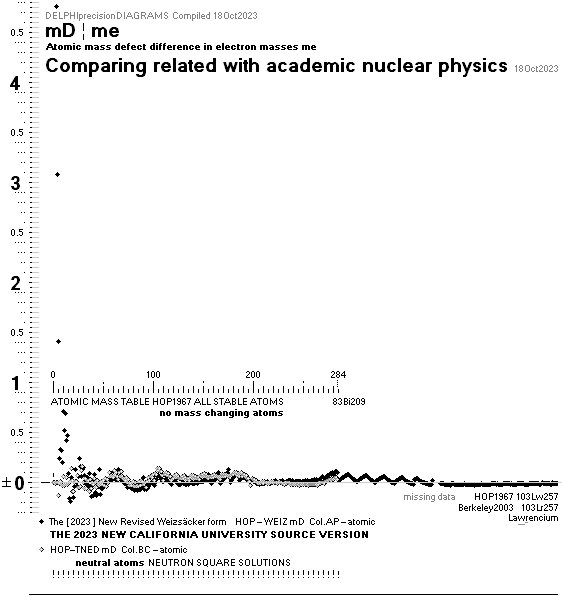
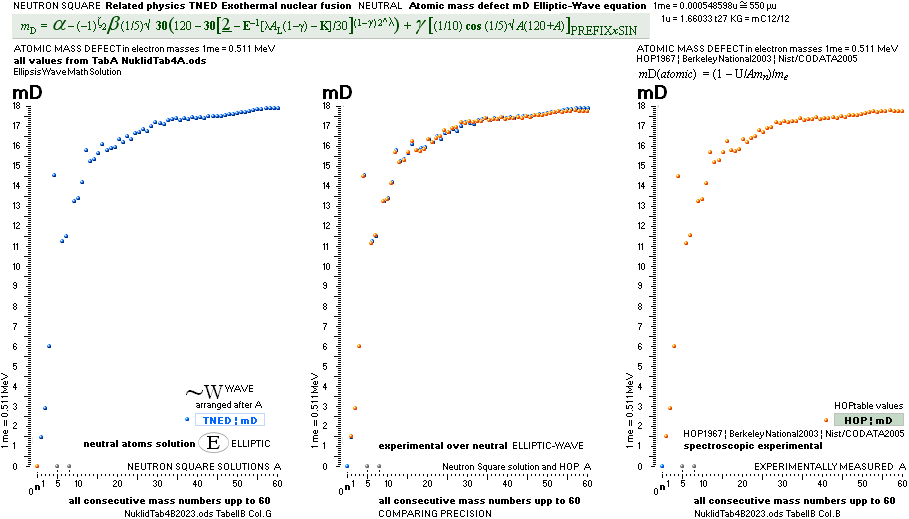
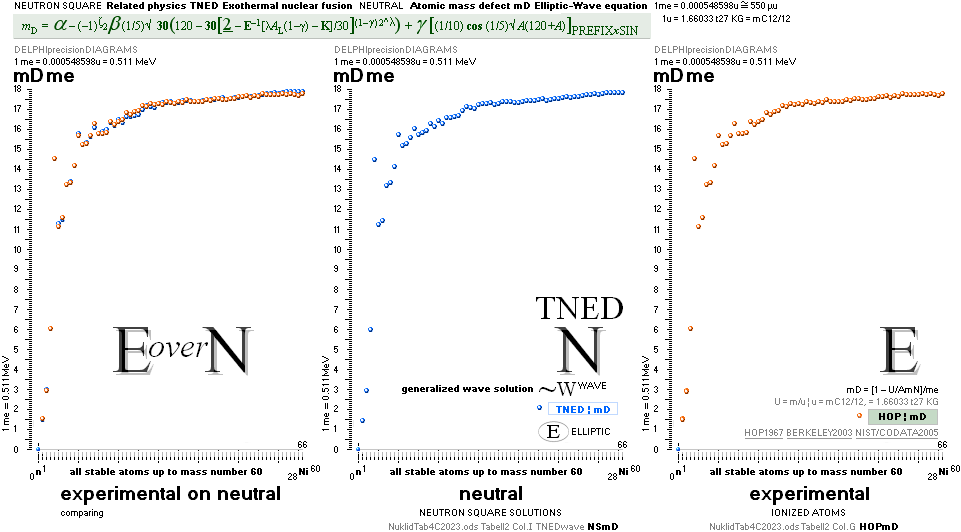
NuklidTab4A2023TableA: NuklidTab4A2023.ods
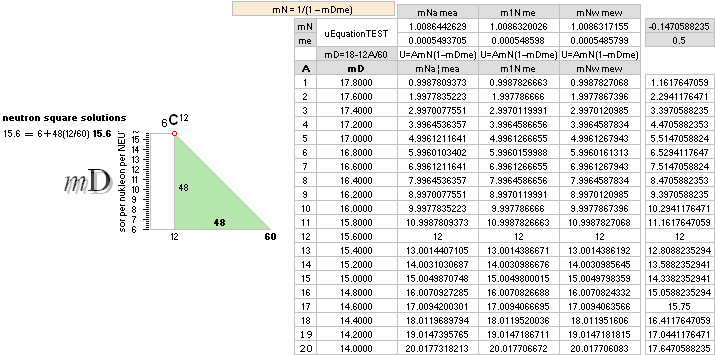
¦ EC ¦ BaseGroupMassNumbersEquation
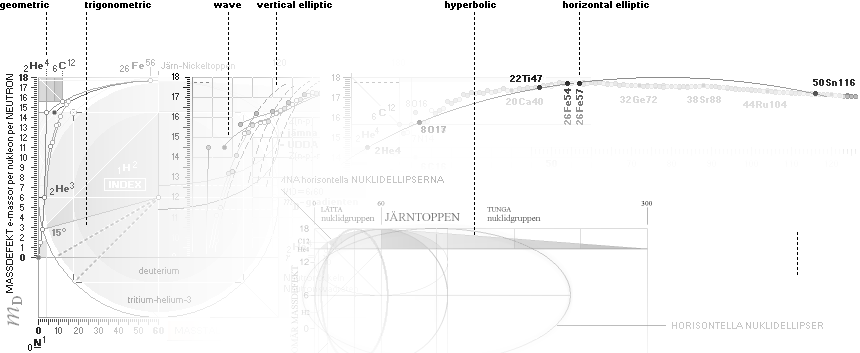
THE NEUTRON SQUARE ATOMIC MASSES UPP TO A=60 —
from MproblemSolved the original MsWORKS 4.0 that Microsoft suddenly blocked from computer usage 2008 — see
ExcelOpenOfficeCALCULUSprograms, TheMicrosoftLIST.
The precision increases with increasing
mass number:
THE TNED DEDUCED ATOMIC MASS DEFECT mD IN ELECTRON MASSES PER
ORIGINAL NEUTRON same as total mD divided with mass number BETWEEN EXPERIMENTAL
AND TNED CALCULATED TAKES A LARGEST VALUE
ON THE HYDROGEN ATOM WITH —0.057 e-MASSES
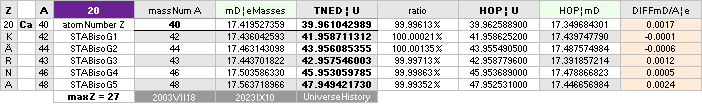
and a U-ratio 100.00313%. 20Ca42 HAS THE LOWEST
DIFFERENCE WITH —0.0001 e-MASSES, and a
U-ratio 100.00021%.
NOTE: 19K40 — not stable — Kalium-40 är betaMinusAktiv
Mass number 40 for the Potassium
element 19K40
has in the HOP table no remark suggesting otherwise than a stable nuclide — abundance 0.0001181.
However other contemporary sources Van Nostrand’s Encyclopedia
claim beta-activity with a half life of 1.3 billion years. Not included here as a stable atom.
Masstal
40 för Kaliumindividen 19K40(17.27) har i HOP-källan ingen anmärkning i tabellen som
antyder annat än att nukliden är stabil. Tre sådana anges.
I avdelningen Nuclear Physics i HOP, första kapitlet General Principles of Nuclear Structure (s9-7sp2n) påstås
emellertid att ”For odd Z there
are never more than two stable isotopes”. Man skulle då kunna misstänka att
HOP-tabellen längre fram i verket är felaktig på den punkten, eller att
författaren är fel ute. Emellertid, kontroll i en annan källa, Van Nostrand´s
Scientific Encyclopedia (Ed5 1976 s491-515) anger i en liknande tabell att
Kaliumindividen 19K40 är –betaaktiv med en livslängd på 1.3 miljarder år.
Innan denna detalj uppmärksammades togs
HOP-tabellens specifikationer för givna varför individen 19K40
också finns medtagen i tabellreferenserna här som en icke instabil markerad
isotop. Den är dock inte upptagen i
nedanstående gruppredovisning av alla stabila isotoper upp till masstal 60.
Om vi tittar efter på masstal 40 ser vi den också, tydligt. Den avviker något
från övriga i en liten dipp som därmed avslöjar att ovanstående citerade
referens tycks ha visst fog för sig. Vi får därmed frånräkna individen 19K40
för vidare, men låter den stå kvar i tablåerna som ett exempel på precisionen
för de stabila nuklidernas del.
Editor2003VII15
THE
GENERAL COLLECTED NEUTRON SQUARE SOLUTION on elliptic and trigonometric-wave
equations — from [2003] the MsWORKS original NuklidTab4.wks ¦
Windows 95, Windows XP, Windows Vista
NuklidTab4A2023.ods TableA
From NuklidTab4.wks 2008 — finally solved
for OpenOffice and Microsoft EXCEL. Explicitly designed for ZERO interrupts: no
errors allowed.
ALL STABLE ISOTOPES UP
TO ATOMIC NUMBER 27 — max mass number 60 — the NeutronSquare
horizontal square side scale
— From A=60 and up the precision —
elliptic equations — becomes more demanding. See FusionEllipsesCompleteExplanationInTNED.
There is however (CompCALu2023)
a simpler »general hyperbolic»
solution for all the 60+ atomic masses (TheWaveFunction
¦ WAFO). In TNED from the NeutronSquare it
exposes a »still excellent fit» to the experimentally measured values. We can
study these for a first rough comparison, and then test the further more
precise determined values. Largest-Smallest mD e-mass per A neutron difference
for this hyperbolic case is +0.00173 -0.00459 — taken for all the (NuklidTab4A2023.ods)
HOP table listed nuclides — including the unstable ones from mass number A=60
to A=257.
The details behind the above
illustrated content is specified in
Geometric ¦ AtomicMassDefectEquation
2003: The NeutronSquare — nuclear
mass connects definite nuclear size.
With the advent of the atomic
mass defect equation in (TNED) related
physics and mathematics
mD in number of electron masses me
U atomic weight in Dalton — u = 1.66033 t27 KG —
units: 1u = m[6C12]/12
A mass number — number of originally
primary neutrons — conventionally »number of neutrons n and protons p»
mN neutron mass in Dalton 1.0086652
me electron mass in Dalton 0.000548598
mN/me = 1838.623545838670 = 1818 + 18 + 2.623545838670..
conventional
atomic weight in UniverseHistory denoted U in u units
the extensive 1967 available (HANDBOOK OF PHYSICS,McGraw-Hill, ed.
1967) HOP table
on experimentally measured atomic
masses did expose
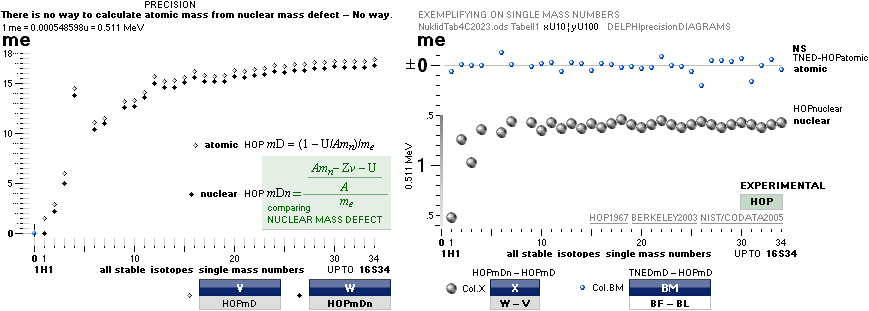
Uncertainty:
±1 pixel, or ca 1 electron mass (±½) me = 0.000548598u = 0.511 MeV per mass
number A ¦ 1 u = 1 Dalton = mC12/12 = 1.66033 t27 KG
definite regular
connections to elliptic
equations of the form
explaining the coherences as exposed:
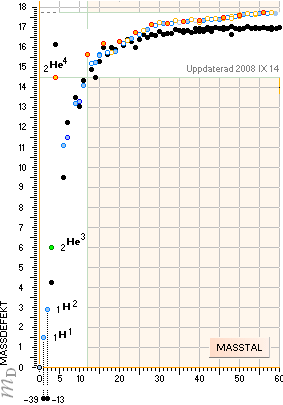
— In this scale [6 pixel colored dots, MAX 18 electron masses Atomic Mass Defect Equation per mass
number A on equation (5)
¦ DiffREF]
no direct difference is visible between the HOP table experimentally measured
and the TNED
atomic mass defect equation wave form (WAFO) and its elliptic
equated values
(see TheActualDifferencesHOP/TNED
¦ ExperimentalErrors):
![]()
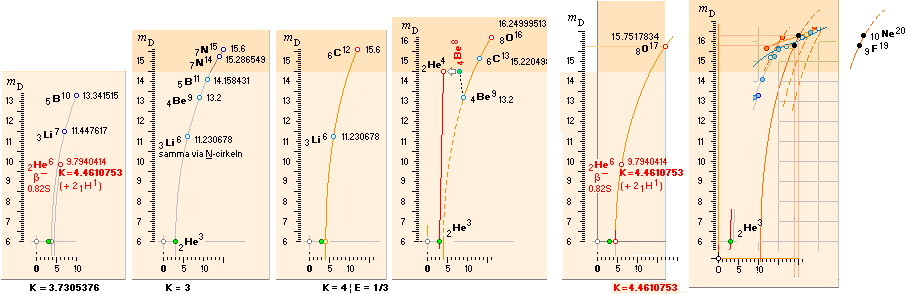
Beginning from
The Cosmological Fundamental Neutron 0;0 (Planck
constant h = mcr):
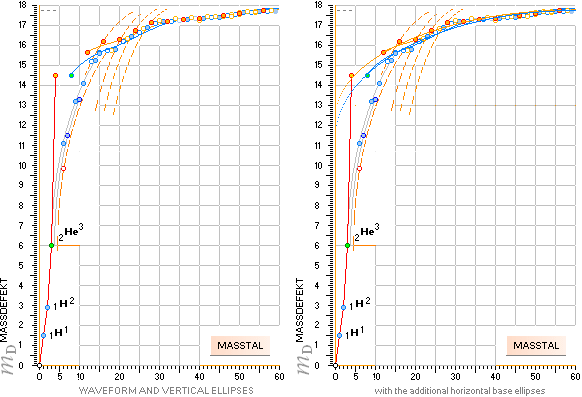
Left: The ordered (exothermal, beginning from Dmax) fusion paths
for the stable nuclide formation according to the TNED discovered Neutron
Square paragonic geometric mathematics. The (dotted) vertical ellipsis arcs
point to (exemplified) a corresponding nuclide’s atomic mass defect for a given
mass number through the neutron square’s special elliptic functions.
Right: Uppermost are included the two fundamental mean ellipses along
with their modification in the form of a general in explicit connecting and
containing also A
hyperbolic expression (CompCALu2023)
connecting the nuclide chart also to the heavy part of the chart further from
mass number 60 Wave Equation (WAFO)
TNEDComparingMAC:
TheoryExperiment
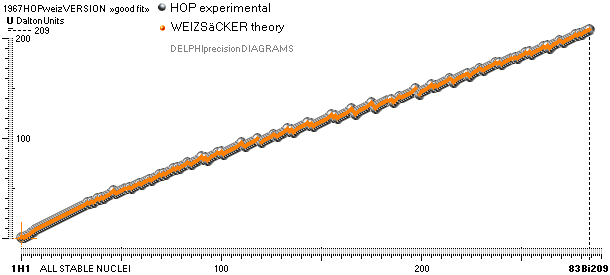
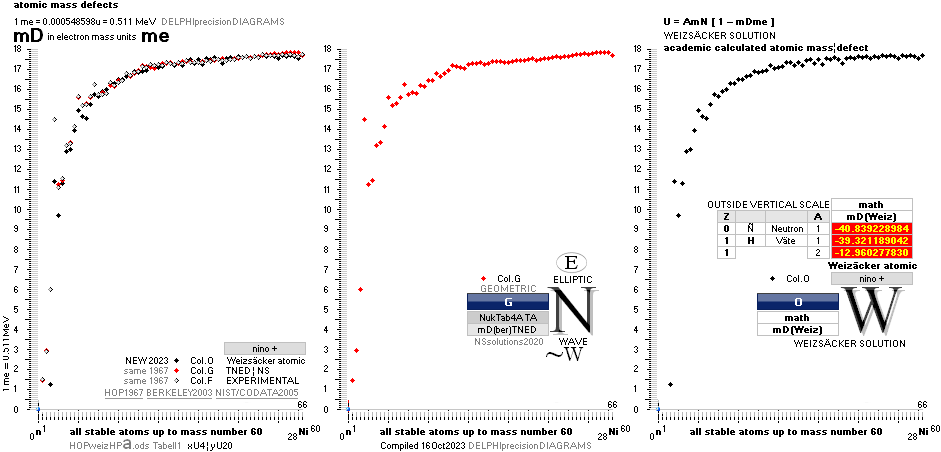
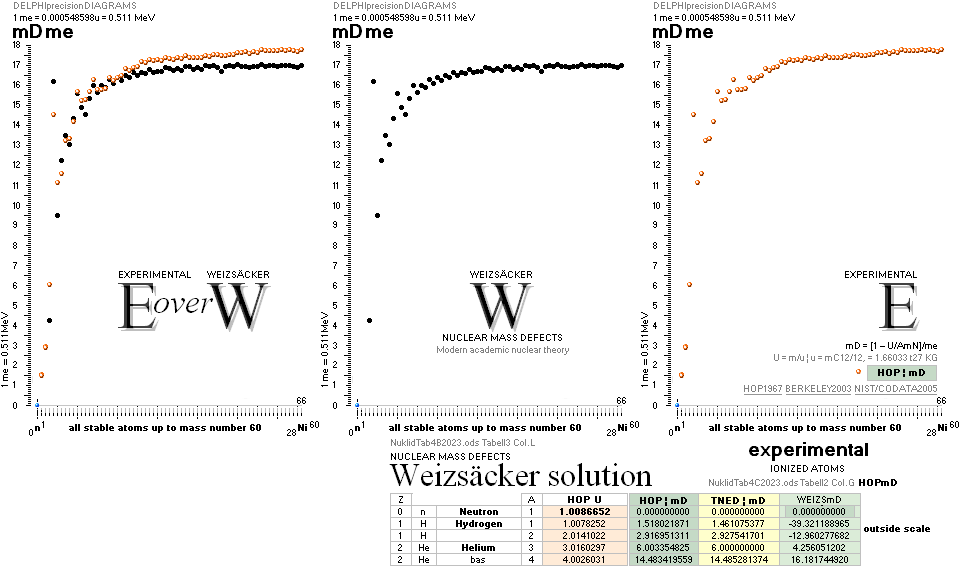
COMPARING TNED RESULTS WITH WEIZSÄCKER VERSION HOP1967¦FM1975
heavy differences
in established corridors
See also the comparing modern academic MAC
theory values in Comparing
TNED/MAC:
Black dots:
Values
from the Weizsäcker equation solution transformed to the TNED mD value form
through the general (colored dots) experimental transfer equation
— The U value
is the atomic (”molecular”) weight value in Dalton units (1Dalton = 1u = 1.66033 t27 KG)
in the different available tables on atomic masses.
— The
Weizsäcker solution is the modern academic concept of nuclear physics based on
nuclear — not atomic — mass defect. It uses the idea of the atomic nucleus as a
uniformly charged liquid drop consisting of discrete neutron n and proton p
smaller drops. See the Weizsäcker equation more detailed in THE WEIZSÄCKER
EQUATION DETAILS.
Colored dots:
The
experimentally measured values. Especially in the first part of the chart, the
Weizsäcker values differ vastly — apparently exposing a direct inaccurate
chosen idea of the atomic nucleus. The Neutron
Square calculated TNED values in
this scale have no visual difference from the colored dots.
— In our era of
experimental physics, the neutron was first discovered 1932
by James Chadwick (ordinary atomic physics fact book information).
— The above
divulging vast Weizsäcker values show no such divulging visibility if viewed in
comparing U-values, the graph below. And because the mD solution never was
searched for in modern corridors — never represented — also what we know the above mD divulging chart was lost in modern quarters.
Uweiz: »the ideal perfect match» — TNEDcomparingMAC
One way of
viewing the Weizsäcker theoretical comparing experimental (THE WEIZSÄCKER
EQUATION DETAILS). Comparing experimental
with theoretical on the Weizsäcker form in U values,
no essential difference is seen, the diagram above. Viewed on the established
term ”nuclear binding energy” the differences exposes more visibility. See for
example TheTakadaDiagram2006.
That figure has the following text, recited here:
The above figure is not the Takada reference — see
the actual Takada figure in TheTakadaDiagram2006:
“ As seen
in the above figure, the Weizsaecker-Bethe mass formula can reproduce well the
experimental data for a wide range of nuclei. We can therefore conclude that the liquid drop model is
enough valid in nuclei.”,
Internet Seminar MICROSCOPIC WORLD –3– The World of
the Atomic Nucleus, Dr. Kenjiro Takada 2006.
The following
Takada consenting HOPweizQuote ”good approximation” certifies the educated established scientific community merits:
” A number
of fairly good semiempirical binding-energy or mass functions have been
constructed which give a good
approximation to the true masses over the wide range of both stable and
unstable nuclei in terms of relatively few empirical constants. Perhaps the most simplest such formula is
that of Weizsäcker: .. (2.1)”, HOP1967, p.9—8.
With no further references — unknown NS solutions — also no objections could be made on the quoted and
established sentences. We just had to conceive them as »provably accurate».
See further
contextual in FIBAPO ¦ NScredit: an explaining inclusive overview.
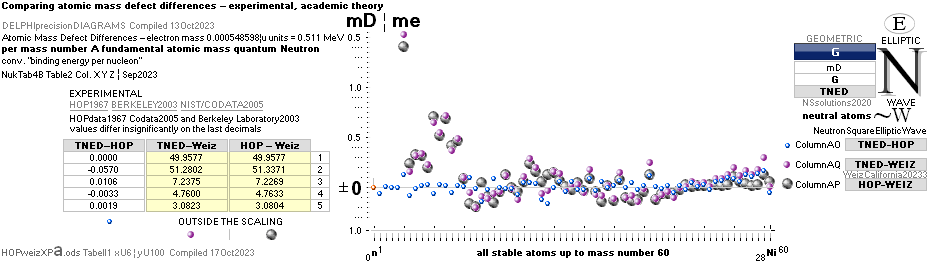
mDweiz: EC
Differences in
atomic mass defect electron masses per mass number
MODERN ACADEMY IS OUTCLASSED BY TNED
— related physics and mathematics
NuklidTab4A2023.ods Table4 — Columns KMS ¦ 1.00 e-mass = 0.511MeV
The large first
1967¦1975
Weizsäcker differences.
The Element isotopes from 1H1 to 4Be9 are left out in the diagram above as the
Weizsäcker values anyway lie far beyond the vertical scale. The reason: The
idea behind the Weizsäcker solutions — charged liquid drop mathematics — have
no corresponding atomic nuclear connection — says TNED on its near and
close contact to the experimentally measured values, directly from the neutron
and up. Only when the atom grows larger and heavier, that difference decreases.
See further definite proofs [Jun2023] in THE ATOMIC NUCLEUS.
The Weizsäcker value equative calculations are accounted for in the separate
spread sheet HOPweizXP.ods Table1. Its equative
details are exposed as quoted in The Weizsäcker
details.
Resurrection11Sep2023 ¦ AtomicMassDefectEquation
AtomicMassDefect: Equation
•
mD =
(1 — U/AmN)/me
•
U = AmN(1 — mDme)
THE ATOMIC MASS DEFECT
Related physics and mathematics: not represented in
the modern academic teaching system.
And it can never
be.
No way.
— IF the reader believes that this production is some kind
of ATTEMPT to be welcomed into modern
quarters, the reader has perfectly lost control of the reading capability. It
will never happen. We could equally hope to have an aquarium at home with gold
fishes in it, on the level of all the Pacific Ocean’s water. It will guaranteed
never happen.
— So what is your aim?
— Live and see. Don’t die.
— That’s easy for you to say, not living in the middle of an
ongoing war; ”don’t die”.
— I hear you. But I’m not talking to the body, only the
mind: it can never die. No way.
TNED — related physics
The atomic mass defect ..
•
Each atom is built of — weighed (U) on — a (mass) number of A neutrons,
each with mass mN = 1.0086652 ¦ u: 1u = 1.66033 t27
KG, the
atomic mass unit;
The TNED related A-Z chart.
White: all stable atoms.
See related deduction from DeducingTheAZ.
•
The atomic weight (U=m/u) of an atom will always be smaller than AmN = A
· mN, because
•
building an atom from lighter units needs work = energy, and
•
it is the atom self that must offer some of its mass for a (an
exothermal, giving out) mass-energy emission during the work.
•
This energy work for building a physical atom never exceeds or even
touches 18 electron masses (Never18);
•
It is (see further below in The 18e
capital) the mN/me structure
mN/me= 1818(»Central Massif») + 18(»Work Capital»: TheNeutronSquare that (2003)
revealed the whole story) + (mN/me — 1836 = 2.624 =»lubricant») electron
masses which is responsible for that mathematics;
See further on
AtomicMassDefectEquation: Explaining the U equation
mD ¦ AtomicMassUnit
Deduction:
THE IMPORTANCE OF relating the mass defect quantity to the fundamental ATOMIC MASS QUANTITY (FAMQ)
The Neutron mass mN:
Atomic mass defect is the Planck energy E=hf=mcr/t=mc2 mass-energy Waste Work needed to build the atom from its number A mass number neutron masses — as related. MDa = (m[WASTE]=E/c2)/u in Dalton units: 1u=1Dalton = m(6C12)/12, = 1.66033 t27 KG
MDa=mD(u)
= AmN — U, atomic mass
defect in U Dalton units, 1u = m(6C12)/12 = 1.66033 t27
KG
mD(AmN) =
(AmN — U)/AmN, atomic mass defect
per mass number A neutron masses mN
mD(me) =
[(AmN — U)/AmN]/me, atomic mass defect
in electron mass
units me
per mass number A
neutron [
fundamental atomic mass quantum ] mass mN
=
AmN[(1 — U/AmN)/meAmN]
mD = (1 — U/AmN)/me, atomic mass
defect
in electron mass
units me
per mass number A
neutron masses mN;
1 me = 0.511 MeV = me·c²/[e=1.602 t19C · T6]
mDATOMIC =
(1 — U/AmN)/Ame, atomic mass defect PER MASS NUMBER ¦ sometimes used in UH for comparison
EXAMPLE:
mD(u¦6C12) = 12·1.0086652
— 12.0000000 = 12(1.0086652 — 1 = 0.0086652)
mD(AmN) =
(0.0086652)/(AmN) = 0.008590759
mD(me) =
(0.0086652)/(meAmN)
mD =
(0.0086652)/(0,000548598·1·1,0086652)
= 15.659479896 — establishedNominal¦experimentalHOP
NOTE THAT DIFFERENT PRECISION VALUES ON THE
DIFFERENT CONSTANTS GIVE DIFFERENT END QUANTITIES. AS THERE YET IS NO COMMON
HIGH PRECISION DEFINITION OF NUCLEAR MASSES PER KILO GRAM — STANDARD UNIT KG —
WE ARE STILL FIGHTING A PRECISION BATTLE LOOKING FOR »BETTER CONFIRMATIONS».
=
(1 — U/AmN)/me
•
mD = (1 — U/AmN)/me
•
U = AmN(1 — mDme)
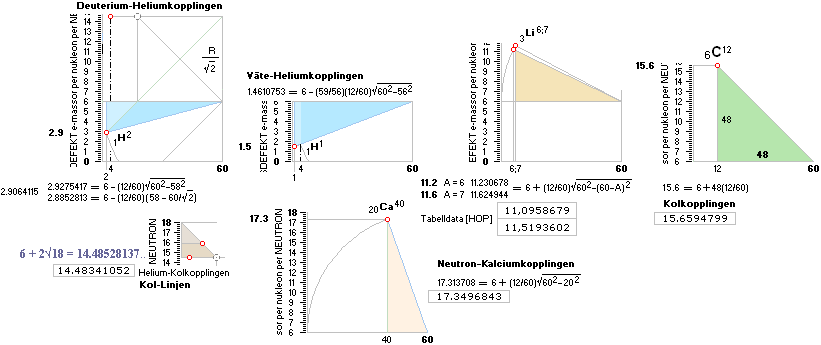
The NeutronSquare gives (directly
trigonometric)
15.6
The NeutronSquare general[‡]
elliptic-Wave (from
NuklidTab4A2023.ods
TableA) gives
15.739439592 (NuklidTab4A2023.ods Table5 A6):
NeutronSquare average
(15.739439592 + 15.6)/2 =
15.669719796.
As an average:
That is a good precision average
original neutron mass defect score for the 6C12 atomic mass relative the
experimental
15.659479896
The rounded difference in electron
masses: 0.01.
The mD average gives a corresponding
comparing experimental
U = AmN(1 — mDme) =
11.999932005.
The atom with the highest building
atomic mass defect work score is represented by Iron (experimentally) 26Fe58
with mD = 17.759142302 electron masses
(1me = 0.000548598u = 9.1085 t31 KG =
0.511 MeV);
18me → max 9.198 MeV.
Atomic and nuclear mass defect
concept — what modern academy missed: onsetting the deduction to the periodic
system: the (deduction) cube analogy, with further ..
WHEREAS the neutron in any case is a
compressed or »sequestered» version of the hydrogen atom, so containing the
proton, the hydrogen nucleus, one can relate any atom on a sum of neutrons
alone. That the atom — then — consists of a nucleus detailing electron masses
to the surrounding cover makes nothing to the point in the internal charge
distribution. It is the same. The only difference consists of the mass defect
(beta transfers) associated with the atomic rebuild on its fitness to the
actual atomic harmony. It was precisely this detail (here in UH) which showed
up its importance for the (TNED)
discovery of (the hidden and forgotten) electron resonances in (the
astoundingly simple) deduction of the elements periodic system via the Kepler momentum.
See THE
KEPLER RESONANCES.
Reason for choice of e-mass
units — atomic mass defect mathematics — related physics
ATOM DYNAMICS — matter in general (The ATOMIC
CHEMISTRY RANK) — entirely is built on the electron
resonances in the Periodic
System. It is the related, deduced and described resonant
areas 2-8-18-32-50- .. -2n² with the related resolution 2-6-10-14-18-… that are
the featuring Commander DoIt. When the atom switches building, these resonant
conditions must be preserved, if we are talking stable tables. That
preservation apparently involves that the resonances — also, in deep — must be
related to the basic fundamental atomic nucleus. That is: The
neutron — which contains the proton-hydrogen atom — as the
most elementary UNSTABLE
nuclide — with a mass defect zero. Shorter:
— The resonant conditions (basic nuclear STRUCTURE) must be — also
dynamically beginning from the neutron — related to the hydrogen atom with its
atomic mass defect (mD) when the neutron decays and becomes stable (THE
NUCLEAR CHARGE BASICS 2).
— If hence the mass defect is uniformly
expressed in e-masses of the neutron mass, one receives directly a quantified
measure on ”distance
to resonant stability” with the neutron-proton-hydrogen atom
as a foundational basic reference. The higher mass defect, the more stable
building, analogously more work to build the construct and thereby harder to
break it up. Consequence, related physics (HIGHESTmD):
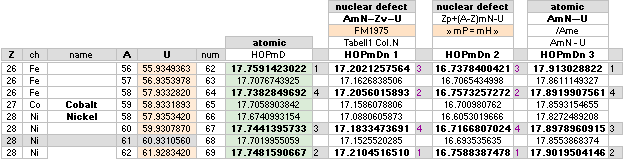
— Iron 26Fe56 ¦ U = 55.9349363 ¦ mD =17.759142 has the highest mass defect score in the max18 scale related physics atomic masses.
— Compare the different modern academic
related highest score elements in (Swedish original) TWO DIFFERENT MASS DEFECT REFERENCES:
And more related, here in English from HIGHESTmD.
— There is no correspondence between
the two different domains. The academic highest (nuclear mass
defect) is 28Ni62 with 17.176463 — with the 26Fe56 in a third
place on 17.168214. The term atomic mass defect
has no representation in the academic society.
Decay, fusion and fission — in preserved resonant
orders: basic related nuclear surface n-p-structure.

The top
spinning atomic nucleus’ inherent spin cannot be removed or stopped. No way.
Any attempt to
do so will promptly result in either a complete ±e structural
annihilation. Or a corresponding (water splash) split on lighter nuclide
elements, of shorter or longer lifetimes.
— But
(intelligent) experimentation can divulge (such impossible) inner structural
properties (Collisions between spin polarized protons 1979 ¦ 1987), helping us to pinpoint the essentials. This story has
exactly that type of credit to thank for.
Preserved electron resonances
— demands a defined characteristic nuclear n-structure and a characteristic
defined p-structure: nuclear
structure:
nuclear surface charge
electric displacement — decays, fusions and fissions
ON BUILDING ATOMS in preserving the
electron resonances for all the elements nuclides, in accordance with the
ordered deduction of the elements periodic system, the nucleus always must
preserve a definite basic configuration related to the neutron-proton basic
aggregate. Such a condition guarantees that the nucleus always is exacting
distributions of separate n-p-individuals in their preserved properties. As
this, primary through the neutron decay, basic configuration is determined by
the resonant numbers in the corresponding integer electron area numbers
(2-8-18-32-50- .. -2n²), with groups and sub-groups, there is apparently a
definite whole number order by which THE NUCLEAR DYNAMICS adjusts in all possible decays,
fusions and fissions.
The 18e capital ..
The MAXmD = 18e capital — basic np-structure
mN/me = 1.0086652/0,000548598 =
1838.623545838670 = 1818 + 18
+ 2.623545838670..
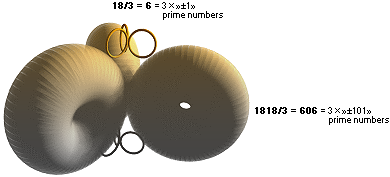
THE EIGHTEEN BODIES
—— 3 × 606 + 3 × 6 + k = 3 × »±303» + 3 × »±3» + k = 3 × 3 × »± 101» + 3 × 3 × »± 1» +
k ¦ prime numbers
WHOLE NUMBER PRESERVED RESONANT SOLUTIONS
mK = 1818, MAXmD = 18 ¦ MPcKärnteorin.doc
28Jun2003 Author’s reference
Mass is taken
from the atom for rebuilding energy
The atom’s performance in the
rebuilding work (m→γ) of producing other atoms needs an
available marginal working mass-energy expendable waste capital (MAXmD). That
capital secures that no causing havoc happens on the central preserved and
protected nuclear massif (mK) on its inherent status of maintaining a stable
nuclear and atomic dynamic function. The expendable (MAXmD) mass must have the
capability in preserving the basic neutron deduced Planck ring (h=mcr) structural
symmetry. That symmetry optimizes a greatest possible symmetric deadlock, a safe
guard, on the entire ring structure of ±β-charges, certifying the
structure does not collapse (PRIME).
— WHEREAS this deduced Planck ring
structure in TNED is founded on a ±β pairwise
(fractal hollow ring) symmetry (NuclearStructure ¦ NuclearMassprinciple ¦ TheFractal TNEDPlanck RingStructure),
it is obvious that the fix nuclear mass (mK, total mass m minus MAXmD) must
be whole number based (PeriodicSYSTEM)
with respect to this pairwise symmetry — if a guaranteed exact balance is to be
certified.
— The expendable mass must be based on
an INTEGER
nuclear ring symmetry.
— By further divisible consequence the
integer part in the fix nuclear mass (mK, so) must be based on an odd integer —
a prime number. A prime number blocks any further split. The prime number
thereby determines the limit for the nuclear symmetry (type 1818 ÷ 18 = 101,
prime number; can only divide by itself for a resulting integer — nuclear
divisions, fragmentation).
The expendable MAXmD mass thereby becomes a function of the named
pairwise ±β electric charge symmetry on the topmost rings structure’s 3-
number (HOW3),
so that one receives MAXmD = x(2[±β]×3)=6x: 6 · N=3 = 18: there never was
not much to chose on.
See also THE simple BASIC
NEUTRON MASS PROVISION MATHEMATICS.
npSTRUCTURE: CAPITAL18e
Basic related nuclear physics n-p surface charge structure
the neutron and
its decay
———————————————
TNED
NUCLEAR CHARGE BASICS ¦ TNEDbacisPlanckRingStructure ¦ TNED
nuclear charge basics 2
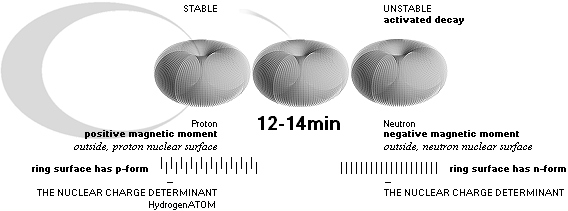
The neutron has by consequence a certain negative nuclear surface prominent electric displacement (The
Neutron Decay) which by consequential known theory through the nuclear top spin gives the neutron a corresponding known
negative magnetic moment. When the neutron undergoes decay the displacement is
changed, ending on the proton characteristic positive electric displacement
with a positive magnetic moment. These moments are well empirical documented,
and mark one of the TNED nuclear toroid morphology’s most important
confirmations (Toroid Nuclear Electromechanical Dynamics, TNED).
See more related nuclear structure
details in
TNED RELATED NUCLEAR ATOMIC
PROPERTIES
If a p-nucleus ( ¦ ) grows optional
with enclosing n-masses ( |
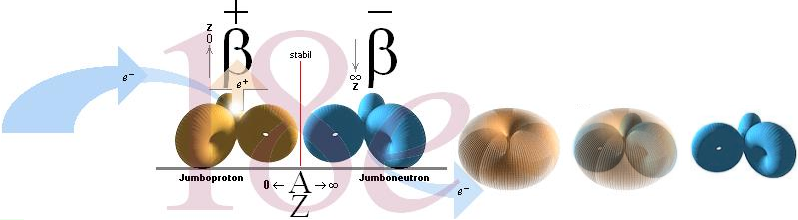
) on each p-ring, ideally a Zn-p-Zn nuclide (a »JumboNeutron»
.. | |
| ¦ |
| | ..),
we can directly see why the resulting edifice sooner or later must lose its
characteristic original p nuclear type charge property: The neutron masses
forces the edifice to withdraw (»bury») the (±e structural) positive
β-displacement.
The same (with slower destructive force) would hold for a »JumboProton»
(Z=[A=»n+p+n»]/3) of the form type
Zn-Zp-Zn = .. | ¦ | | ¦
| |
¦ |
| ¦ | ..
= Z(n-p-n): the p-type gradually loses impact over the n-type, and the nuclear
structural balance is again compromised — IF we are talking »overall uniformly
distributed charge». If not, this alternative would apparently suit the best
fit in preserving a given Zp (on still larger nuclei), type nX-Zp-nX.
If the p-nucleus on the other hand grows evenly with a minimum of
enclosing n-mass type (»A=Z»),
n¦..1111111111..¦n .. ideally a n-Zp-n
nuclide (a »JumboProton»), we see equally easy that the nuclear surface charge
— n-p-p-p..p-n = n-Zp-n — is forced to grow in magnitude with the integer
number Z>1; The original p-nucleus also here loses its original hydrogen
atom harmonics.
An ideal (Z=[A=»n+p»]/2) p-type
nucleus Zn-Zp = Z(n-p) = .. | ¦ | ¦ | ¦
| .. would be the
theoretically preferred natural choice on building heavier atoms from lighter
atoms for a preserved exact balance — provided
the nuclear ring structure did have a »uniformly distributed electric displacement»
all over the nuclear surface.
— It hasn’t.
— For the most low atomic nuclear charge
atomic number Z-values, the ideal Z(n-p) type »is The Man». In the empirical
table values, it holds practically up to the stable Calcium 20Ca40. After that,
further on the stable Calcium isotopes, the Z(n-p) type is definitely done; On
the following nearest stable Scandium atom 21Sc45, the threshold has already
been passed: more n:s than p:s. And so the more n:s than p: s continues to grow
with still heavier atoms — remember we talk Structure here.
A balanced growth on heavier atoms apparently (in
some way) favors the named type
nX-Zp-nX.
The NUCLIDE MAP (AZ) IN TNED
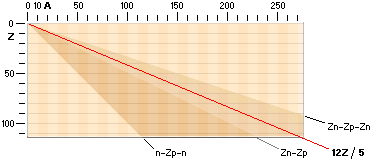
Nuclide static
average value line A=12Z/5
crosses with good approximation the nuclide field (white in the AZ-map below).
In that region we find the natural element’s stable atoms. While the n-p
structure demands certain structural padding with growing atomic number Z, some
of the atomic elements will expose isotopes. That is, atoms with same Z (same
chemical properties) but different masses (different n-p structural paddings on
their mass number A).
Atoms with same mass number (A) but different atomic numbers (Z) are
called isobars.
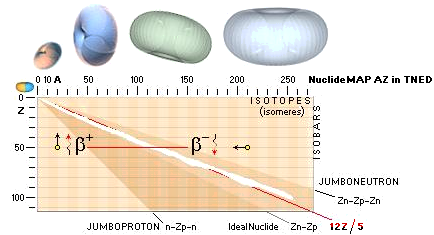
Beta decay
emission mechanism
The term beta relates to both
polarities of the electron mass — the normal negative electron mass and its
positive spouse, the positive electron: the positron
Jumboneutrons
at right and Jumboprotons at left. The Jumboneutron is to heavy for its atomic
number Z. It strives to get rid of one or several electron in order to
switch down to a more stable position further down in the chart via a higher Z
padding. The Jumboproton is to light for its Z. By burning off +β
rings, or alternatively pull in one (or several) electron, it can reduce its Z
and there by emigrate further up for a more correct balance. The AZ-chart
also includes limits for atoms of a hard (nuclear) instability (their mass
number A changes during the decays — in general from 83Bismut209). The
description of the hard unstable atoms are (what we know) more demanding and
will apart from the short description below on Radioactive mathematical
physics not be mentioned further in this presentation (it needs a whole
document, not to say several — see article links Swedish edition in Radioactive
Decay’s Physics).
Atoms classified apart from the stable
atoms are unstable atoms. These are either soft or hand unstable. The soft
unstable are classified as beta (Greek b, β) unstable atoms. They become
stable by electron transactions, give or take: their mass number (A) is
preserved. The hard unstable are classified as the alpha unstable atoms (often
emitting a Helium-4 nucleus — and more). They become stable by nuclear decay,
nuclear emission or nuclear split. In the AZ map below only the beta unstable atoms
are addressed.
AZchart: NuclideStaticAverage ¦ DeducingTheAZ
THE related RADIOACTIVITY MATHEMATICAL PHYSICS IN TNED
Short description
According to TNED all unstable atoms change towards a stable and balanced state and condition is described by the two type ranks below:
(K) – Ehf18 = K = (K1 + K2 – (m→γ) + β0γm ) – Ehf18
................ beta decay, beta unstable atoms, beta
nuclides
(K) – Ehf1818 = K = (K1 + K2 – (m→γ) + T0γm ) – Ehf1818 ............. nuclear decay, nuclear unstable atoms, radio nuclides
the cryptic are explained further through the link Radioactive
Decay’s Physics — these factors explain necessary energy-mass
transactions
Is the Ehf1818 neutrino regulated radio component removed out of the nuclear
reaction law, one receives only the expression of a stable
nuclide, which not is the radio case. By that reason we safely know that the
neutrino influences play an important role for the decay of the radio nuclides.
But the related TNED theory — it is extensive — is not found in modern
quarters: TNED
is not welcome there. No way (npStructure).
— Each
radionuclide has its own specific neutrino spectrum. And different radio
types therefore does not interfere in their specific decays. Beta decays on the
contrary have mutual influences because the neutrino emissions are of the same
electron-positron type for all beta decaying atoms. Their corresponding
energies can therefore also be generated by electron oscillations. In the radio
nuclide case on the contrary, the neutrino levels are isolated from electron
generating effects (Planck’s structural constant).
Understanding
the world we live in: We get credit for trying.
DEDUCING THE ATOMIC A-Z NUCLEAR CHART
A = n + p A = mass number — number of original fundamental neutron = whole fundamental atomic masses
= n + Z
n = A – Z the actual practically atomic
physical case in any way — Z = the nuclear charge = the atomic electron charge
With the three named nuclear
compositions as delimited by the Jumbo Proton n-Zp-n ¦ »A=Z» nuclide, the ideal
Z(n-p) ¦ Z=A/2 nuclide, and the Jumbo Neutron Z(n-p-n) ¦ Z=A/3 nuclide, the
theoretical region for all possible nuclides is identified by its absolute
limits and borders — with respect to the discussed ring structure in the TNED
toroid nuclear complex:
Theoretical limits of atomic nuclide spectra as related by
nuclear n-p-structure
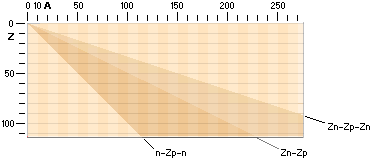
The theoretical
limits for all possible atomic nuclei in a general A-Z nuclide chart, related
physics (Deducing the AZ).
The diagram
summarizes the extreme limits for the Jumbo Proton (n-Zp-n) left bottom and the
Jumbo Neutron Z(n-p-n) right limit in the theoretical nuclide spectrum, as
related. Z denotes atomic number or nuclear charge, A denotes mass number, n
denotes neutron nuclear surface structure type and p denotes proton nuclear
surface structure type, as explained in related nuclear physics (TNED — see Nuclear
STRUCTURE).
DEDUCING THE A-Z CHART
The connection
for the Nuclide static average value line A=12Z/5
is the average mean of the coefficients for the ideal Z(n-p)
nuclides via k=1/2 with Z=A/2, and the Jumboneutron’s Z(n-p-n) nuclides via
k=1/3 with Z=A/3. The average mean becomes [(1/2)+(1/3)]/2=5/12 with Z=5A/12,
or A=12Z/5.
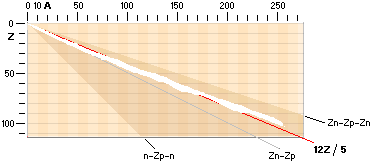
Author’sRef: MPcKärnMatIIIa.doc ¦ MPcKärnMat.doc ¦ MPcKärnMatII.doc
And that is also
the stretch where we find the stable atoms and their surrounding unstable.
TNED BASIC RELATED ATOMIC NUCLEAR PHYSICS
AND ITS MATHEMATICS
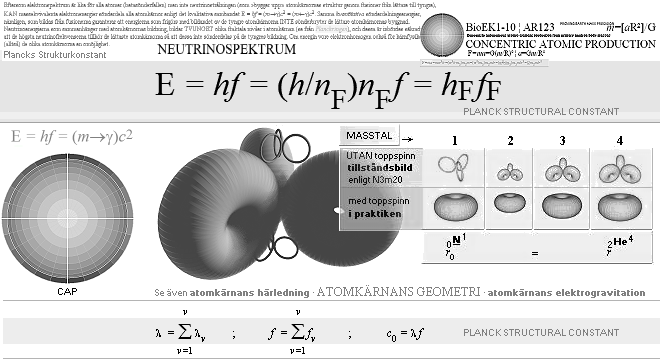
THE PLANCK STRUCTURE CONSTANT, THE ATOMIC
MASSES, CAP and
CWON
Building heavier from lighter —
basic related atomic nuclear principles (Neutrino Spectrum ¦ The Plateaus)
Because electron spectrum is the same for all atoms (beta decays) but not the neutrino radiation (the high frequency radiation associated with the building up of the atomic nuclear structures by fusions from lighter to heavier, our Sun the nearest source) mass equivalent electron energies CAN decompose all atomic nuclei according to the qualitative connection
E = hf = (m→γ)c2 = (m←γ)c2
The same quantitatively decomposing energies namely
E = hf = (m→γ)c2 ≠ (m←γ)c2
gravitation, the
atomic nucleus, is not a particle — BackGROUND
¦ Atomkärnan ¦ CompareQm
gravitation
cannot be compressed, it is already standing on a zero
gravitation is
not light — ENERGY related physics LAW
light is not a particle,
but its matter
generated induction quanta makes it convenient to express as »traveling
photons»:
MAX PLANCK WAS RIGHT, ALBERT EINSTEIN with associated WAS
WRONG
mass is not
light — EXPERIMENTAL CONFIRMATIONS
mass cannot be
created, only destructed, and has hence quality light energy equivalents
ENERGY EXCHANGE — NO MASS CREATION. See also The
Particle Proof , Swedish edition.
formed by the fusions guarantee that the released (exothermal) energies in building heavier from lighter does not break or destroy the already built lighter atomic nuclei structures. The neutrino energies connecting the atomic nuclei buildings, so constrainedly form different fractal (Planck structural constant) levels (see from Planck Ring 1). These so safely built levels become inwardly secured in that the highest neutrino frequencies belong to the lightest atomic nuclei, attesting that these in no way are compromised on credit of the heavier built structures.
— So in deducing the atomic
masses, we must properly find the different exothermal fusion paths to each
single individual atom and its nucleus, its actual atomic mass defect, and do
the end experimental testing theory mass calculations from there, for any
further heavier building: the Neutron
Square, Neutron Squatre solutions as noted, is
our only reference.
• The only possible spontaneously exothermally energetic way for the basic fundamental atomic nucleus the neutron to build heavier atoms, unequivocally must begin from a maximum closed packed neutron nuclei state, a Dmax (certifying the nuclei lie inside each others nuclear barriers, leading to direct fusion): A compact high gravitating neutron mass spherical body. Thereby a process begins of a gravitational (light’s gravitational dependency) managing concentric atomic production (CAP). The gravitational potential is the least in the center, analogously the local divergence c the highest, and thereby the fastest decaying neutrons: the atomic building process begins at the centre, ends on the surface (CWON). It is fast and releases humongous amounts of exothermal energy (Element formation’s two basic nuclide groups).
See a basic complementary basic description in
WAFO: Neutron Square solutions ¦ BaseGroupMassNumbers
Neutron Square solutions
THE MEAN ELLIPTIC WAVE FUNCTION
TNED NEUTRON SQUARE SOLUTIONS ATOMIC AND NUCLEAR RELATED MATHEMATICAL PHYSICS
Excerpts from the original
Swedish edition (2003 — Toroid Nuclear Electromechanical Dynamics)
———————————————
Atomic
Weights ¦ THE NEUTRON SQUARE PARAGONIC BODY ¦ Mean
Ellipsis for Even and Odd ¦
THE
WAVE FUNCTION ¦
The
wave function’s explanation ¦
WaveFunction
Description
Explain:
The (EE) neutron square horizontal elliptic basic equation
y = 0.2[5x(120 + 5x)]½
has a simple (PREFIXxSIN) associated damped
cosine wave functional form:
y = cos 0.2[5x(120 + 5x)]½
A = 5x accounts for the graphical scale adoption.
This PREFIXxSIN neutron square elliptic associated damped cosine wave functional form has the followingt close general first provisional connection to the experimentally measued atomic masses:
—————————————————————————————————————
mATOM = Uu ¦ atomic weight U = mATOM/u, u atomic mass unit m(6C12)/12 named 1 Dalton (1.66033 t27 KG)
—————————————————————————————————————
The plateaus: THE ORIGIN
OF THE GENERAL NEUTRON SQUARE WAVE EQUATION 2003
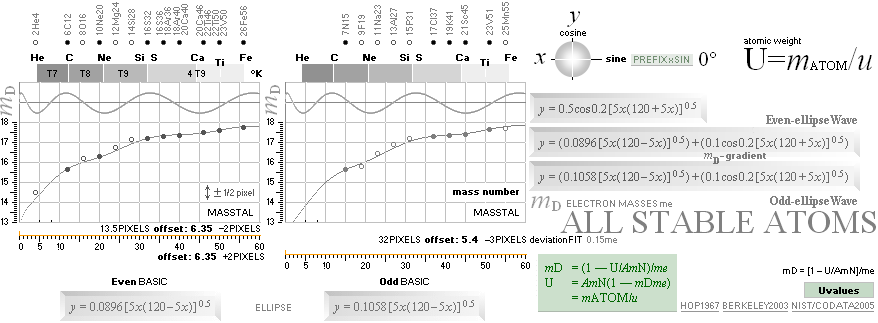
The open rings
apart from the curved elliptic wave line follow (VerticalElliptic)
specific fusion paths by vertical elliptic arcs with specific mass defect
equations. See further (Sw., ed original) in Wave function’s Explanation.

The Plateaus:
DeducisngTheAZ ¦ PLATÅERNA
— The plateaus¦ NuclearSTRUCTURE ¦ IronTOP
¦ HIGESTmD
Transdlated 22Oct2023 from the original 2003:
Helium plateau
to Carbon plateau
From the Helium Reference the nucleus
captivates a stable state (ideal nuclear). We then conceive the nucleus on (the previously described)
nuclear line for the Z(n-p) type — the nuclide chart’s angled space, right
below the static mean value line (12/5-line). As the nucleus grows with
n-p pair nuclear structures, it however approaches the Jumbo Proton — which
gives instability. Already at the Carbon plateau, a phase shift appears where
stable nuclides can form with the addition of one neutron. Then nuclear fusions
can proceed toward stable nuclides with growing mass defect — more atomic
binding energy — in a slower phase where the additional neutron gives a certain
margin ahead.
Neon plateau to
Silicon plateau
When further neutrons must be added to
fend off the divergence toward a Jumbo Proton condition, the nucleus enters
into the state phase three, the Neon plateau. From this point — the n-Z(n-p)-n
nuclide type — the mass defects growth can occupy an even larger interval
before the nucleus enters stage phase four, the Silicon-Sulfur plateau. From
that point more nuclear neutron structure addition is demanded to bring
(Planck) ring stability to the nucleus
— the 2n-Z(n-p)-2n type nuclides. And
as earlier, this fill promotes an even longer stage and with even lower mass
defect increase before the nucleus enters the final Calcium plateau.
Calcium plateau
From here the nucleus receives a
further neutron dilutive fill in guaranteeing a stable nuclide. We now see the
3n-Z(n-p)-3n nuclide type appear mixed with the 2n-Z(n-p)-2n type from the
previous plateau, and with certain neutron additions. This phase then continues
finally up to the iron top where the 18 limit is reached by the nuclide
individual 26Fe56 (HIGESTmD) — composition 15n-26p-15n,
analogously 2n-26(n-p)-2n. That finalizes the basic nuclear synthesis.
The heavy nuclide group’s Certification
The heavy
nuclide group
Continuing the atomic production —
exothermal (heat
and light emission of the work mass-energy waste in building heavier from
lighter) — from the basic ideal top mass number 60 highest atomic
mass defect point, demands a presence of specifically neutron rich low mass
beta active nuclides, type:
2He6,
3Li8 and 6C16.
Such exothermal provisions are only
possible in a — TNED deduced (CAP)
— situation where atomic production begins from a maximum dense celestial body
of neutrons. With their decay — fastest where the gravitational potential is
the lowest, that is in the body center — close an tight fusion rings are
asserted to bring out heavier atoms from the lighter. Beginning from a
celestial core with a such iron end atomic produced center (no significant net neutron quote in the
center), heavier atoms beginning from neutron decay need a farther
distance (slower decay) from the center, securing a higher rate of neutron
quote — and thereby a much more diverse possible set of also the most heavy
atomic products. However, the fusions agents into the heavier nuclides
(A>60) depend on momentarily produced beta active atoms of the above suggested
type: neutron structure rich nuclei who can complete the near fusion processes
in building the most heavy possible atoms.
This is readily physically
energetically impossible unless all those atoms — during the very short moment
of time in the celestial body’s fusion phase — far less than a second — are
collected in one and the same massive (primary neutron) body. Sharing the
(enormous) massive energy release during the (short) collective fusion phase,
guarantees that the momentarily (normally fast) decaying beta active nuclides
will WAIT on their decays — momentarily FED by the environmental exothermally
fusion emitting mass-energy equivalents. This high surge energy throughput
delay will provides the necessary standard relatable energy calculable support
in building the heavier atoms. The fusion processes as such, are guaranteed as
long as the involved fusion ring agents are situated inside each others nuclear
potential barriers (meaning:
inside each others circumscribed spheres) — where the close nuclear ±e
structure sucks and takes care of the spontaneous fusions.
See further from
BaseGroupMassNumbers:
EllipticWaveEquation: — up to mass number 60 —
WAFO
THE GENERALIZED WAVE-ELLIPTIC
FORMULA
The originally 2003 compiled Elliptic-Wave
equation for the light atomic nuclide chart up to mass number 60 — see NuklidTab4A2023.ods TabellA
THE BASE GROUP MASS NUMBERS NEUTRAL ATOMIC MASS DEFECTS
— from the original works in MsWorks ¦ which Microsoft suddenly and abruptly denied computer access to after a rude Microsoft update on a Windows Vista computer (Aug2008) — supported by bought jurisdiction.
” .. the foundation of freedom, justice and peace in the world”. World Jurisdiction Enterprise. Mississippi 1820.
Unless there are other here unknown candidates:
ONLY WAY TO DEDUCE EXPERIMENTALLY
CONCORDANT ATOMIC MASS VALUES IS TO TRACE THE ATOM’S ACTUAL FUSION TRACK FROM
ITS SET OF ORIGINAL NEUTRONS and the two different nuclear structural types
of ±e electric displacement:
the neutron nuclear structural n type and the proton nuclear structural p type
| | | | | | | | | | | | | | | | | | | | | | .. | ¦ | ¦ | ¦ | ¦ | ¦ | ¦ | ¦ | ¦ | ¦ | ¦ | ¦ ..
with all their possibly
relatable combinations, see Nuclear
STRUCTURE in related physics
— its trace of atomic mass defects, forming the foundation in building heavier from lighter
Mintrusion2008: Base
With the Microsoft 2008 intrusion into the pending work,
also followed a blockade of a possible transfer code to other spread sheet
programs (Microsoft EXCEL and its imitating OpenOfficeCalc). The Microsoft
MsWORKS inhibited corresponding mod/REST operators had been compromised:
Microsoft EXCEL has other routines. See describing example in EOOK — and some additional ”Microsoft
EXCEL features” in the following text and cell code examples. Through these
”Microsoft modification” the MsWORKS original was blocked. Until the recent
attempts (Aug2023) to find a solution, the work made no progress. This is the
solution.
THE LIGHT atomic NUCLIDES
FROM A=1 TO A=60¦64
G0—isoA0 — BaseGroupMassNumbers —
from Z
Basic Group — the group base mass number A for the given atomic number Z — valid up to Z=30, light nuclide chart only (Amax=64):
The role of Z
here is only of an organizing manner, according to the basic observations on
the Elliptic
and wave coherences on the HOP experimental values
A =
[2Z+Zmod2 + (Zdiv[17+ 5(1–Zmod2)])(2×round[(Z–8+Zmod2 )/ 2(6–Zmod2)])]=A’
+ (1–ζ6)(–1)Zmod2(A’–Z)mod3 [1–(Zmod3)div2]
ζ 6 =
INT[1–(abs[Z0/6–1] – [Z0/6–1])/2]
................................... Zζ = .. ,111,101,91,81,71,60,50,40,30,20,10,00,–1–1,–2–1,–3–1, ..
OPEN OFFICE CALC SwedishVersion:
=
HELTAL(1-(ABS(Z/6-1)-(Z/6-1))/2)
A’
OPEN OFFICE CALC SwedishVersion = R3:
= 2*Z + REST(Z;2) + (HELTAL(Z/(17
+ 5*(1-REST(Z;2)))))*(2*AVRUNDA((Z-8+REST(Z;2))/(2*(6-REST(Z;2)))))
+(1-HELTAL(1-(ABS(Z/6-1)-(Z/6-1))/2))(–1)^(REST(R2;2))*(REST(R3 - R2;3) * (1 -
HELTAL(REST(R2;3)/2) ))
ALL IN ONE CELL ONLY:
=
2*Z+REST(Z;2)+(HELTAL(Z/(17+5*(1-REST(Z;2)))))*(2*AVRUNDA((Z-8+REST(Z;2))/(2*(6-REST(Z;2)))))
+((1-HELTAL(1-(ABS(Z/7-1)-(Z/7-1))/2)))*(-1)^(REST(Z;2))*(REST((2*Z
+ REST(Z;2)+(HELTAL(Z/(17+5*
(1-REST(Z;2)))))*(2*AVRUNDA((Z-8+REST(Z;2))/(2*(6-REST(Z;2))))))-Z;3)*(1-HELTAL(REST(Z;3)/2)))
Proof:
![]()
NuklidTab4A2023.ods Table4 —— verified ¦ Author’sREF:
MPcKärnMatIIIa.doc 9Aug2003 —— algorithm: just the atomic number
Z plus »integer selector algebra»
APART FROM THE BASE GROUP, THERE ARE FIVE MORE
ISOTOPIC GROUPS:
NuklidTab4A2023.ods TableA
How it did develop —
all based on and from the (2003)
sudden discovery of the Neutron Square apparently universal paragon geometrical
mathematics
on comparing atomic masses
from known tables: elliptic equations, simple wave form adoptions — whole
numbers selector algebra
(Swedish
original ..)
Analysen visar att bestämningen av en nuklids massdefekt
helt och hållet betingas av dess underliggande sammansättning, dess fusionsväg.
Känner man denna och kan återföra nukliden på mera elementära grundnuklider får
man en specifik massdefektsekvation.
..
Det innebär att hela nuklidkartan byggs upp som en händelse (som en organism
som breder ut sig): varje nytt steg beror av sammansättningarna i föregående
och kan beräknas om man känner nämnda. Av den anledningen, speciellt med tanke
på grundnukliderna upp till Helium (första fasen) finns inte den typ av
”kärnekvation” som den moderna akademin försöker uppställa.
..
Man måste dela in nukliderna i olika grupper, en huvudgrupp med underliggande
isotopgrupper som följer händelsevägen via växande massdefekt upp till
Järntoppen. Därefter, från Järntoppen mot nuklidkartans slut, följer en annan
typ av analys med avtagande massdefekt. Man måste emellertid också känna
samtliga instabila (betaaktiva) nukliders massdefekter och hur de bildas innan
en fullständig beskrivning kan ges. De betaaktiva nukliderna spelar nämligen en
(delvis) stor roll i bildningen av de tyngre nukliderna.
Men ingen sådan övergripande (betaaktiv)
analys har ännu utförts i UniversumsHistoria (Okt2023).
Hela nuklidkartan blir (alltså) en karta
över hela den kosmiska händelsehistorien.
..
Mass number
A generally for all basic nuclides (from the Helium reference) up to the Iron
group (part of the original draft):
A =
A0
+ AEvenFromCarboOddFromNitro
+ AcorrectionBelowCarboNitroPreference
A =
[2Z+Zmod2
+ (Zdiv[17+ 5(1–Zmod2)])(2×round[(Z–8+Zmod2 )/ 2(6–Zmod2)])]=A’
+ (1–z 6)(–1)Zmod2(A’–Z)mod3 [1–(Zmod3)div2]
Author’sRef: MPcKärnMatIIIa.doc ¦ MPcKärnMat.doc ¦ MPcKärnMatII.doc
isoGROUP 1
The actual isoA¦1-5 cell equations are written out as follows.
—
What we know (related physics, NS solutions):
—
There is no ”overload version” possibly defining the isotope mass numbers in a
given atom — before, prior, that specific atom base nucleus has been made/specified.
So, when the math to this analyze was first
projected (from 2003), it had to be on »all the tabled stable isotopes
together», in order for the author to have any the smallest chance in »luring
out the hidden matrix». That is: all based on already existent atomic mass
tables. Below is an illustrating extract of the actual providing data needed
(the neutron square content) in order to succeed on the exercising quest.
G1— isoA
A =
isoAJ
+ isoAU
+ isoAneon
:
AisoA1 =
isoJ1
+ isoU1
+ isoAneon
isoJ1 = (A+2)g10(1–Z0mod2)
isoU1 =
A(Zmod2) – b[round([(Z–7)/2]mod8/8) – 1]
isoAneon = ζ1(1– γ10)(Φ[(1+λ)Z + λ(1–Amod2)] + (1–λ))
b = (Zmod2)nollarViaJämna(round[(nHe+1)/4] – 1)mod2 (INT[(nHemod7)/7+7/8])
nHE = (Z – 6 – Z0mod2)/2
Φ = INT[1–(|a2–1| –
[a2–1])/2]
......................................................... A|Φ= 11,41,61,90,101,121,151,161,190
a2 = (Amod9)(Zmod9) ......................................................................... A|a2= 11,48,618,90,105,1218,1542,1656,190
λ = INT[1–(abs[Z0/03–1] – [Z0/03–1])/2] ........................................ Zλ = .. ,51,41,31,20,10,00,–10,–20, ..
ζ1 = INT[1–(abs[Z0/01–1] – [Z0/01–1])/2]
........................................ Zζ = .. ,51,41,31,21,11,00,–10,–20, ..
γ10 = INT[1–(abs[Z0/10–1] – [Z0/10–1])/2] ......................................... Zγ = .. ,111,101,90,80, .. ,20,10,00, ..
OpenOfficeCalc:
isoJ1 = (A+2)*Gma10*(1-REST(Z0;2))
isoU1
= (A*REST(Z0;2)-AVRUNDA(REST(((REST(Z0;2)*(N24+0*REST((AVRUNDA((nHe+1)/4;0)-1);2))*HELTAL(OM(nHe<0;nHe;REST(nHe;7))/7+7/8))*(Z0-7)/2);8)/8;0)-1)*(REST(Z0;2)*(N24+0*REST((AVRUNDA((nHe+1)/4;0)-1);2))*HELTAL(OM(nHe<0;nHe;REST(nHe;7))/7+7/8))
N24 = OM(N25<0;N25; REST(N25;2)) ¦ the
tricky MicrosoftEXCEL-OpenOffice cell code removal of the original MsMORKS mod operator
consequence
N25 = (AVRUNDA((nHe+1)/4;0)-1)
= (AVRUNDA((((Z0-6-REST(Z0;2))/2)+1)/4;0)-1)
IT MEANS THAT VERY WELL IN MsWORKS 4.0 — BLOCKED FOR FURTHER
WINDOWS USE BY MICROSOFT 2008 IN WINDOWS VISTA — WE CAN CALCULATE THE G1
A-VALUES from the Z inputs IF WE STILL HAVE A TYPE WINDOWS XP COMPUTER ALIVE
accepting MsWORKS. — BUT HOWEVER NO SO DIRECTLY in Microsoft Excel and
OpenOffice — »the Excel imitator». To do so also in these latter programs, we
need to add the above N25 and N24 in two demanding separate cell rows. See the
complete — but very lengthy — solution in last rank 5 below. See NuklidTab4A2023.ods
Table4, Rows 2 and 3.
isoAneon
= Theta1*(1-Gma10)*(L10*((1+Lda)*Z0+Lda*(1-REST(A;2)))+1-Lda)
L10 =
HELTAL(1-(ABS(K10-1)-(K10-1))/2)
K10 =
REST(A;9)*REST(Z0;9)
isoAneon
= Theta1*(1-Gma10)*(HELTAL(1-(ABS(REST(A;9)*REST(Z0;9)-1)-(REST(A;9)*REST(Z0;9)-1))/2)*((1+Lda)*Z0+Lda*(1-REST(A;2)))+1-Lda)
=
(HELTAL(1-(ABS(Z/01-1)-(Z/01-1))/2))*(1-(HELTAL(1-(ABS(Z/10-1)-(Z/10-1))/2)))*(HELTAL(1-(ABS(REST(A;9)*REST(Z0;9)-1)-(REST(A;9)*REST(Z0;9)-1))/2)*((1+(HELTAL(1-(ABS(Z0/3-1)-(Z0/3-1))/2)))*Z0+(HELTAL(1-(ABS(Z0/3-1)-(Z0/3-1))/2))*(1-REST(A;2)))+1-(HELTAL(1-(ABS(Z0/3-1)-(Z0/3-1))/2)))
b = 6-A
nHE = (Z0-6-REST(Z0;2))/2
ΦFi = HELTAL(1-(ABS((REST(A;9)*REST(Z0;9))-1)-((REST(A;9)*REST(Z0;9))-1))/2)
λLda = HELTAL(1-(ABS(Z0/3-1)-(Z0/3-1))/2)
ζ1 = HELTAL(1-(ABS(Z/01-1)-(Z/01-1))/2) ¦ Theta1, i.e., Tzeta (ϑ not explicitly available 2003 in this production)
γ10 = HELTAL(1-(ABS(Z/10-1)-(Z/10-1))/2) ¦ Gma10
OpenOfficeCalc
¦ Tabell4 G1:
G1.1:
(A+2)*Gma10*(1-REST(Z0;2))+
(A*REST(Z0;2)-AVRUNDA(REST(((REST(Z0;2)*(N24)*HELTAL(OM(nHe<0;nHe;REST(nHe;7))/7+7/8))*(Z0-7)/2);8)/8;0)-1)*(REST(Z0;2)*(N24)*HELTAL(OM(nHe<0;nHe;REST(nHe;7))/7+7/8))+
Theta1*(1-Gma10)*(HELTAL(1-(ABS(REST(A;9)*REST(Z0;9)-1)-(REST(A;9)*REST(Z0;9)-1))/2)*((1+Lda)*Z0+Lda*(1-REST(A;2)))+1-Lda)
G1.2:
(A+2)*(HELTAL(1-(ABS(Z/10-1)-(Z/10-1))/2))*(1-REST(Z0;2))+
(A*REST(Z0;2)-AVRUNDA(REST(((REST(Z0;2)*(N24)*HELTAL(OM(((Z0-6-REST(Z0;2))/2)<0;((Z0-6-REST(Z0;2))/2);REST(((Z0-6-REST(Z0;2))/2);7))/7+7/8))*(Z0-7)/2);8)/8;0)-1)*(REST(Z0;2)*(N24)*HELTAL(OM(((Z0-6-REST(Z0;2))/2)<0;((Z0-6-REST(Z0;2))/2);REST(((Z0-6-REST(Z0;2))/2);7))/7+7/8))+
(HELTAL(1-(ABS(Z/01-1)-(Z/01-1))/2))*(1-(HELTAL(1-(ABS(Z/10-1)-(Z/10-1))/2)))*(HELTAL(1-(ABS(REST(A;9)*REST(Z0;9)-1)-(REST(A;9)*REST(Z0;9)-1))/2)*((1+(HELTAL(1-(ABS(Z0/3-1)-(Z0/3-1))/2)))*Z0+(HELTAL(1-(ABS(Z0/3-1)-(Z0/3-1))/2))*(1-REST(A;2)))+1-(HELTAL(1-(ABS(Z0/3-1)-(Z0/3-1))/2)))
G1.3:
(A+2)*(HELTAL(1-(ABS(Z/10-1)-(Z/10-1))/2))*(1-REST(Z;2))+
(A*REST(Z;2)-AVRUNDA(REST(((REST(Z;2)*(N24)*HELTAL(OM(((Z-6-REST(Z;2))/2)<0;((Z-6-REST(Z;2))/2);REST(((Z-6-REST(Z;2))/2);7))/7+7/8))*(Z-7)/2);8)/8;0)-1)*(REST(Z;2)*(N24)*HELTAL(OM(((Z-6-REST(Z;2))/2)<0;((Z-6-REST(Z;2))/2);REST(((Z-6-REST(Z;2))/2);7))/7+7/8))+
(HELTAL(1-(ABS(Z/01-1)-(Z/01-1))/2))*(1-(HELTAL(1-(ABS(Z/10-1)-(Z/10-1))/2)))*(HELTAL(1-(ABS(REST(A;9)*REST(Z;9)-1)-(REST(A;9)*REST(Z;9)-1))/2)*((1+(HELTAL(1-(ABS(Z/3-1)-(Z/3-1))/2)))*Z+(HELTAL(1-(ABS(Z/3-1)-(Z/3-1))/2))*(1-REST(A;2)))+1-(HELTAL(1-(ABS(Z/3-1)-(Z/3-1))/2)))
G1.4:
(A+2)*(HELTAL(1-(ABS(B5/10-1)-(B5/10-1))/2))*(1-REST(B5;2))+
(A*REST(B5;2)-AVRUNDA(REST(((REST(B5;2)*(N24)*HELTAL(OM(((B5-6-REST(B5;2))/2)<0;((B5-6-REST(B5;2))/2);REST(((B5-6-REST(B5;2))/2);7))/7+7/8))*(B5-7)/2);8)/8;0)-1)*(REST(B5;2)*(N24)*HELTAL(OM(((B5-6-REST(B5;2))/2)<0;((B5-6-REST(B5;2))/2);REST(((B5-6-REST(B5;2))/2);7))/7+7/8))+
(HELTAL(1-(ABS(B5/01-1)-(B5/01-1))/2))*(1-(HELTAL(1-(ABS(B5/10-1)-(B5/10-1))/2)))*(HELTAL(1-(ABS(REST(A;9)*REST(B5;9)-1)-(REST(A;9)*REST(B5;9)-1))/2)*((1+(HELTAL(1-(ABS(B5/3-1)-(B5/3-1))/2)))*B5+(HELTAL(1-(ABS(B5/3-1)-(B5/3-1))/2))*(1-REST(A;2)))+1-(HELTAL(1-(ABS(B5/3-1)-(B5/3-1))/2)))
G1.5: only variables: B5=Z and B2
(B4+2)*(HELTAL(1-(ABS(B5/10-1)-(B5/10-1))/2))*(1-REST(B5;2))+
(B4*REST(B5;2)-AVRUNDA(REST(((REST(B5;2)*(B2)*HELTAL(OM(((B5-6-REST(B5;2))/2)<0;((B5-6-REST(B5;2))/2);REST(((B5-6-REST(B5;2))/2);7))/7+7/8))*(B5-7)/2);8)/8;0)-1)*(REST(B5;2)*(B2)*HELTAL(OM(((B5-6-REST(B5;2))/2)<0;((B5-6-REST(B5;2))/2);REST(((B5-6-REST(B5;2))/2);7))/7+7/8))+
(HELTAL(1-(ABS(B5/01-1)-(B5/01-1))/2))*(1-(HELTAL(1-(ABS(B5/10-1)-(B5/10-1))/2)))*(HELTAL(1-(ABS(REST(B4;9)*REST(B5;9)-1)-(REST(B4;9)*REST(B5;9)-1))/2)*((1+(HELTAL(1-(ABS(B5/3-1)-(B5/3-1))/2)))*B5+(HELTAL(1-(ABS(B5/3-1)-(B5/3-1))/2))*(1-REST(B4;2)))+1-(HELTAL(1-(ABS(B5/3-1)-(B5/3-1))/2)))
It works.
ALL THE ABOVE IN THE FIRST ATOMIC NUMBER CELL. THEN COPIED
FOR EACH NEW CELL WITH EXTENDING COLUMN PREFERENCE UP TO Z=28, MASS NUMBER 60.
— It is amazing that OpenOffice can handle this — because
OpenOffice in other tracks is sometimes readily horrible. .. cannot write in
capitals .. program insists on converting to literals, or vice versa, in
forming constant names .. suggests .. cannot leave the user alone STOP
SURVEILLING SPYING AND MAKING CHANGES TO MY WRIT .. shut the fuck up .. .. we are trembling on the brink .. cannot
select last color .. cannot read bookmarks backwards .. looses text caret
position in text documents .. why was I born .. COPYING an OpenOfficeCalc to a
new document it is IMPOSSIBLE to get rid of the Original’s 178 Constants by
erasing That Table, these must be erased manually, one by one, through repeated
several button clickings .. why not let us die directly .. and when pressing Ctrl+F3 in finding out
what Constants are there, no key shortcut exists to get into the actual Box:
must Click to get there .. no single keyboard operations, no sir .. but the
Design is OK .. don’t touch me .. so in time we learn how to escape the
OpenOffice Orcs .. taking care not to be too creative ..
— On the other
hand:
THE OpenOffice FREEWARE AT PRESENT 2023 IS APPARENTLY THE
BEST HUMANITY CAN OFFER.
Yes, we CAN get things done .. but OpenOfficeProgram Man ..
you’ve got to fix the engine .. jises ..
The other 4 groups
are somewhat simpler.
The70: BGMN
70 stable isotopes
NuklidTab4A2023.ods Table4 —— verified mapping ¦ the five atomic group
finalized with their corresponding atomic and mass numbers
OPEN OFFICE HAS NO HERE KNOWN FUNCTION
BY WHICH TO LEAVE A CELL BLANK IF ITS RESULT IS IRRELEVANT to the author: OPEN
OFFICE DEMANDS A VALUE 0. SO TO SHOW THE RESULTS IN ONLY VALID POSITIONS, NO
OTHER WRITS, WE MUST MAKE A NEW TABLE USING THE CELL CODE FOR EACH POSITION: if B2 = 0 then ShowNothing: Sw.:
Om(B2=0;””).
AND THE MOST STRANGE OF ALL IN OPEN OFFICE: IF ANOTHER OPEN
OFFICE DOCUMENTS IS OPENED with the first showing its cell code in the inbox
THAT TEXT DISAPPEARS ON OPENING THE NEW OPEN OFFICE DOCUMENT.
It just proves on the many examples in OpenOffice, that IT
is a more or less MicrosoftOffice2000 Imitation — with SOMETIMES extremely
careless and left out functionality. Microsoft Office (2000) has no such.
— The OpenOffice case: Most likely poor partial
functionality because the programming personnel cannot solve the programming
functions: If they could, there would be no problems. Meaning: it is the
programming language — or the programming person not understanding what a
computer is for: the functionality is anyway incomplete. Say I’m wrong.
— But the design is OK.
A =
isoJ1
+ isoU1
+ isoAneon
AisoA1 = (A+2)γ10(1–Z0mod2)
+ A(Zmod2) – b[round([(Z–7)/2]mod8/8) – 1]
+ ζ1(1– γ10)(F[(1+λ)Z + λ(1–Amod2)] + (1–λ)) ¦ A = mass number in group isoA0, the base group — the structure extends from there:
NuklidTab4A2023.ods TableA
isoGROUP 2
A =
isoJ2
+ isoU2
+ isoN2
— IT
LOOKS LIKE A RAIL YARD WITH SETS, CARTS, SWITCHES, longer or shorter trails,
SIMPLE WHOLE NUMBER ORGANIZATIONS ..
isoJ2 = γ16(1 – γ27)(1 – Z0mod2)(2Z0 + round[(2Z0 + 1)/10] – abs(1 – 2Z0mod32) + [1 – 2Z0mod32])
isoU2 = Z0(18/8)ζ8(1 – ζ9)
isoN2 = ζ10(1 – ζ15)(1 – Z0mod2)(2Z0 + 1)
γ16 = INT[1–(abs[Z0/16–1] – [Z0/16–1])/2] ................................... Zζ = .. ,191,181,171,161,150,140, .. ,20,10,00, ..
γ27 = INT[1–(abs[Z0/27–1] – [Z0/27–1])/2] ................................... Zζ = .. ,291,281,271,260,250,240, .. ,20,10,00, ..
ζ 8 = INT[1–(abs[Z0/08–1] – [Z0/08–1])/2] ................................... Zζ = .. ,111,101,91,81,70,60,50,40,30,20,10,00, ..
ζ 9 = INT[1–(abs[Z0/09–1] – [Z0/09–1])/2] ................................... Zζ = .. ,111,101,91,80,70,60,50,40,30,20,10,00, ..
ζ 10 = INT[1–(abs[Z0/10–1] – [Z0/10–1])/2] ................................... Zζ = .. ,111,101,90,80,70,60,50,40,30,20,10,00, ..
ζ 15 = INT[1–(abs[Z0/15–1] – [Z0/15–1])/2] ................................... Zζ = .. ,171,161,151,140,130,120, .. ,20,10,00, ..
OpenOfficeCalc:
isoJ2 =
Gma16*(1-Gma27)*(1-REST(Z;2))*(2*Z
+ AVRUNDA((2*Z+1)/10;0)-(ABS(1-REST(2*Z;32))+(1-REST(2*Z;32))))
isoU2 = Z0*(18/8)*(Theta8*(1-Theta9))
isoN2 =
Theta10*(1-Theta15)*(1-REST(Z;2))*(2*Z+1)
γ16 = HELTAL(1-(ABS(Z/16-1)-(Z/16-1))/2)
γ27 = HELTAL(1-(ABS(Z/27-1)-(Z/27-1))/2)
ζ8 =
HELTAL(1-(ABS(Z/08-1)-(Z/08-1))/2) ¦ Theta8, i.e., Tzeta (ϑ not explicitly available 2003 in this production)
ζ9 =
HELTAL(1-(ABS(Z/09-1)-(Z/09-1))/2)
ζ10 =
HELTAL(1-(ABS(Z/10-1)-(Z/10-1))/2)
ζ15 =
HELTAL(1-(ABS(Z/15-1)-(Z/15-1))/2)
OpenOfficeCalc
¦ Tabell4 G2:
(HELTAL(1-(ABS(B5/16-1)-(B5/16-1))/2))*(1-(HELTAL(1-(ABS(B5/27-1)-(B5/27-1))/2)))*(1-REST(B5;2))*(2*B5
+ AVRUNDA((2*B5+1)/10;0)-(ABS(1-REST(2*B5;32))+(1-REST(2*B5;32))))+B5*(18/8)*((HELTAL(1-(ABS(B5/8-1)-(B5/8-1))/2))*(1-(HELTAL(1-(ABS(B5/9-1)-(B5/9-1))/2))))+(HELTAL(1-(ABS(B5/10-1)-(B5/10-1))/2))*(1-(HELTAL(1-(ABS(B5/15-1)-(B5/15-1))/2)))*(1-REST(B5;2))*(2*B5+1)
isoGROUP 3
A =
isoJ3
isoJ3 = g16(1 – g27)(round([Z0mod2]/4)(2Z0 + abs[round(2Z0/10) – 7])(1 – Z0mod2)
γ16 = INT[1–(abs[Z0/16–1] – [Z0/16–1])/2]
............................. Zγ
= ,191,181,171,161,150, .. ,50,40,30,20,10,00 ..
γ27 = INT[1–(abs[Z0/27–1] – [Z0/27–1])/2]
............................. Zγ
= ,291,281,271,260,250, .. ,50,40,30,20,10,00 ..
OpenOfficeCalc:
isoJ3 =
Gma16*(1-Gma27)*(AVRUNDA(REST(Z;6)/4;0)*(2*Z+ABS(AVRUNDA(2*Z/10;0)-7)))*(1-REST(Z;2))
γ16 = HELTAL(1-(ABS(Z/16-1)-(Z/16-1))/2)
γ27 = HELTAL(1-(ABS(Z/27-1)-(Z/27-1))/2)
OpenOfficeCalc
¦ Tabell4 G3:
(HELTAL(1-(ABS(B5/16-1)-(B5/16-1))/2))*(1-(HELTAL(1-(ABS(B5/27-1)-(B5/27-1)/2)))*(AVRUNDA(REST(B5;6)/4;0)*(2*B5+ABS(AVRUNDA(2*B5/10;0)-7)))*(1-REST(B5;2))
isoGROUP 4
A = isoJ4
isoJ4 = g20(1 – g25)(1 – Z0/2mod2)(2Z0 + 6)(1 – Z0mod2)
γ20 = INT[1–(abs[Z0/20–1] – [Z0/20–1])/2] ................................... Zζ = .. ,231,221,211,201,190,180, .. ,20,10,00, ..
γ25 = INT[1–(abs[Z0/25–1] – [Z0/25–1])/2] ................................... Zζ = .. ,281,271,261,251,240,230, .. ,20,10,00, ..
OpenOfficeCalc:
isoJ4 =
Gma20*(1-Gma25)*((1-REST(Z/2;2))*(2*Z+6))*(1-REST(Z;2))
γ20 = HELTAL(1-(ABS(Z/20-1)-(Z/20-1))/2)
γ25 = HELTAL(1-(ABS(Z/25-1)-(Z/25-1))/2)
OpenOfficeCalc
¦ Tabell4 G4:
(HELTAL(1-(ABS(Z/20-1)-(Z/20-1))/2))*(1-(HELTAL(1-(ABS(Z/25-1)-(Z/25-1))/2)))*((1-REST(Z/2;2))*(2*Z+6))*(1-REST(Z;2))
isoGROUP 5
A =
isoJ5
isoJ5 = g20(1 – g29)round(INT[(Z0mod12)/4]/2)(2B50 + 2[1 + abs(HELTAL[2Z0/7] – 8)])(1 – Z0mod2)
γ20 = INT[1–(abs[Z0/20–1] – [Z0/20–1])/2] ................................... Zζ = .. ,231,221,211,201,190,180, .. ,20,10,00, ..
γ29 = INT[1–(abs[Z0/29–1] – [Z0/29–1])/2] ................................... Zζ = .. ,321,311,301,291,280,270, .. ,20,10,00, ..
OpenOfficeCalc:
isoJ5 =
Gma20*(1-Gma29)*AVRUNDA(HELTAL(REST(Z;12)/4)/2;0)*(2*Z +
(2*(1+ABS(HELTAL(2*Z/7)-8))))*(1-REST(Z;2))
γ20 = HELTAL(1-(ABS(Z/20-1)-(Z/20-1))/2)
γ29 = HELTAL(1-(ABS(Z/29-1)-(Z/29-1))/2)
OpenOfficeCalc
¦ Tabell4 G5:
(HELTAL(1-(ABS(B5/20-1)-(B5/20-1))/2))*(1-(HELTAL(1-(ABS(B5/29-1)-(B5/29-1))/2)))*AVRUNDA(HELTAL(REST(B5;12)/4)/2;0)*(2*B5 + (2*(1+ABS(HELTAL(2*B5/7)-8))))*(1-REST(B5;2))
THE MICROSOFT INTRUSION
(Aug2008) led to so much (sad and weary) trouble. And it was such a release
when (at last Aug2023) the light came through,. The solution here deserves all
thorough attention on that dramatic history’s compressed content:
— »Fuck
you Microsoft2008», the galactic leading prohibiter of a perfectly functioning
MsWorks spread sheet program Aug2008 on Windows Vista: Microsoft2008, destroyer
of MsWORKS mod operator, vandalized in Microsoft EXCEL and so apparently imitated
equally by the free OpenOfficeCalc.
— As it so seems,
the aim was and is to promote a deeper insight into the world business
inducement of appearing to be the best of the best in the world of computer
programming science:
REST(-A;B) ¦ -A mod B
MsWORKS ¦ Division algorithm ¦ Windows Calculator
latest W7 ¦ Borland’s Pascal:
–A
Microsoft (prohibiting further Windows
use of MsWORKS from 2008)EXCEL¦OpenOfficeCalc:
+A
----------------------------------------------
HELTAL(-0.99)
MsWORKS ¦ Division algorithm ¦ Windows
Calculator latest W7 ¦ Borland’s Pascal
–0
Microsoft (prohibiting further Windows
use of MsWORKS from 2008)EXCEL¦OpenOfficeCalc:
–1
----------------------------------------------
Microsoft
Enterprise:
— If you cannot handle mathematics on
the computer level:
— What can you handle?
”Restart your computer or we will do it
for you”.
•
Where is world jurisdiction
— allowing such raging human right
intrusion partying and feasting?
Not
one word human right recognition.
TNED.
WE, the customers, bough the Windows
HUMANITY DEVELOPING products during a period of several decades, BROUGHT the company
UP, buying merchandise for tens of thousands of dollars PER PERSON. How were we
met? A spit in the face, a foot in the back, vandalized text, dictating,
overriding, private computer settings, a controlling and demanding authority of
We-Own-You attitudes — and We have the Deciding power over your computer.
”Pick a time”.
”Fuck you asshole”.
Muzzled. Handcuffed. Bandaged.
”Let’s kick off ..”.
”Was this helpful?”.
”Like us”.
Toying humanity. Openly. Freely.
Muzzled. Handcuffed. Bandaged.
Tagged Cattle.
— Why, and how, in the world do humans
take such shit?
— Or.. They don’t. But no one is
allowed — deeply afraid — to say it.
.. the foundation of freedom, justice and peace in the world
— Where is world jurisdiction — other
than bought bitches to serve Microsoft Enterprise human right intrusions?
Not
one word human right recognition.
Talk about lawlessness and anarchy. It
is gushing.
Microsoft:
— Offer fully freeware Microsoft OFFICE
fully detailed to every single born human being on Earth, from here to
eternity, at no cost,
to be used
freely as Pen, Rubber and Paper. Remove the brakes, and let
humanity DEVELOP on humanity’s provisions and conditions, not Microsoft’s.
Stop killing
humanity.
Stop Human
Trafficking.
IT IS NOT ALLOWED TO USE HUMANITY FOR PROFITING
PURPOSES, NOT BY BODY, NOT BY MIND, INTENTIONALLY OR NOT, NOT AT ALL. ALL FORMS
AND TYPES OF SLAVERY SHALL BE FORBIDDEN.
Where is world jurisdiction? Where is United Nations?
United Nations explicit formulation in UDHR10Dec1948 — and
present Trafficking.
Slavery (Swedish classic encyclopedic)
definition, five words:
— A state of personal noFree.
(Sw., ett tillstånd av personlig
ofrihet).
Human right recognition.
Microsoft. GOOGLE. Police. Prosecutor. Court. World Business Enterprise. Not
one word. Not a sound. Not a hint.
Where not heard:
violence is gushing.
.. the foundation of freedom, justice and peace in the world
Regaining the mod and Rest operators in Microsoft’s New EXCEL and
— the imitating — Open Office Calc
—
NOTE: MICROSOFT Windows Calculator still existed on Windows 7 y2015, same as
the old MsWORKS mathematical division algorithm mod
operator:
— Not
in Windows 10 — several reports on failed calculator in W10 — many cookies
blocking web sites inhibits further free information on the W10 calculator
subject: Still not one word human right recognition. No mentioning. Not a sound. Not a hint.
Just interrupting. Blocking. Demanding cookies consent.
AFTER THE 2008 MICROSOFT REMOVAL/banning OF FURTHER
WINDOWS COMPUTER USE OF MsWORKS
CRUSHING THE CONTINUED COMPILATION WORKS ON THE NEUTRON SQUARE SOLUTIONS RESULTS IN ATOMIC MASSES DETERMINATION
2003—10Sep2023 REVISITING THE
NEUTRON SQUARE MATHEMATICS
The deuced mathematics to the Light Nuclide Group — up to mass number 60
——————————————————————————————————————
This following was the simple spread sheet calculus code we failed (TNEDa0EOOK) to create a corresponding transfer expression for in OpenOfficeCalc (and MicrosoftEXCEL):
The original (2008) in MsWORKS NuklidTab4.wps:
Rest(A;B)
BORLAND’S DELPHI PASCAL: A mod B
for the case of A less than zero (nHe)
Rest(-1;2)
IN MsWORKS
(blocked 2008+ by Microsoft Windows Vista from further computer use)
— AND IN BORLAND’S PASCAL DELPHI
CODE, the mod operator
as derived in basic mathematics
from the DIVISION
ALGORITHM
it reads
= -1
In OPEN OFFICE
and MICROSOFT EXCEL it presents the
result:
= 1
— Why?. Because in Microsoft’s standard Windows Calculator —
latest Windows 7 — the mod operator has the same status as the above mentioned
MsWORKS, Borland Pascal, General Mathematics, basic deduced division algorithm.
AND WE SHOULD TAKE ADVANTAGE OF THIS OPPORTUNITY TO ALSO
UNDERLINE FURTHER IN OPEN OFFICE Swedish Version Here THE STRANGEST OF ALL mathematics CALCULUS PROGRAMMING FEATURE:
OPEN OFFICE ¦ MICROSOFT EXCEL:
Heltal( 0.99) ¦
= 0 INT( 0.99) =
0 ¦ correct
Heltal(-0.99) ¦ = -1 INT(-0.99)
= -1 ¦ incorrect
ONLY PERSONNEL THAT
is occupied by DO NOT CARE MUCH
ABOUT ELSE THAN PROFIT CAN DO THAT. Say. Do correct. No serious scientific.
Intelligence is still OK. Problem is: low on moral.
— » Please Refill. Follow Instructions .. ».
No PHYSICS — AND MATHEMATICS — DEVELOPS
ON SUCH PROGRAMMING SKILLS- RESULTS.
(It smells rotting brains all over the
place ..).
The reason why Microsoft — apparently after MsWORKS ¦ after
Windows 95 — did lose its nerve and fell into other inducements, is not known
here, except for the apparent possibility of leaving the company to less
educated.
correctly
related mathematics
— for developing physics purposes in explicit:
Heltal(±0.99) ¦ = 0 INT(±0.99)
= 0
The INT integer operator takes the integer part of the argument
— an: makes no operation or modification on
the argument,
♦ independent of sign = direction.
IN 2008 MICROSOFT EXCLUDED ALL USE OF MsWORKS — WINDOWS
VISTA — AFTER AN UNANNOUNCED UPDATE, WHICH THE COMPANY REFUSED TO HELP REMOVE
SO THAT WE COULD CONTINUE TO USE MsWORKS SPREAD SHEET PROGRAM.
Microsoft blocked further use. An abrupt interference in and
a direct attack on our work.
— See also The Microsoft LIST. The great interest 2008+ from
Microsoft to educate humanity.
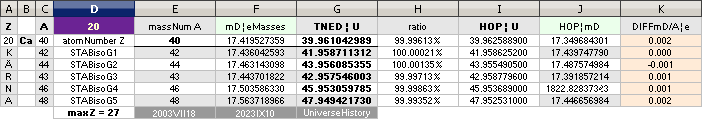
NuklidTab4A2023.ods TableA From NuklidTab4.wks 2008 — finally solved for OpenOffice and Microsoft EXCEL
So .. what has
all this to do with presenting details in nuclear physics? Explain.
THIS WAS THE BOTTLENECK WE COULD NOT SOLVE BEFORE — in
translating the excellent MsWORKS NuklidTab4.wks to a corresponding OpenOffice
— and Microsoft Excel — spread sheet code:
The MsWORKScalc
original — during the actual work around 2008:
SwedishVersion
REST((AVRUNDA((nHe+1)/4;0)-1);2)
English:
REST((ROUND ((nHe+1)/4;0)-1);2)
Namely for the case where nHe becomes = –2;
See BaseGroupMassNumbers:
— if not
properly handled according to the deduced mathematics
the division algorithm —1mod2 = —1 which Microsoft removed from use
2008 and replaced as exemplified above with a +1, then also apparently adopted by other
(OpenOffice) impressed programmers —
the overall
result crashes (like the ”Error41” in the movie PayCheck, Ben Affleck 2003:
perfect machine — blocked from use).
The solution .. finally:
SOLUTION IN OPEN OFFICE (English) — as tested and verified:
IF(nHe<0 ; nHe ; Rest(nHe;2))
.. a separate context breaching type of explaing atomic
physics language ..
Explain ..
PART OF the WHOLE INTENTION BEHIND THE DEDUCED
NUCLEAR/ATOMIC PHYSICS WAS TO USE ONLY WHOLE NUMBERS — PROVING THAT ATOMIC
MASSES THROUGH THE NEUTRON SQUARE INDEED CAN BE DEDUCED LIKE A RAIL YARD WITH
POINTS AND SWITCHES DELIMITING NUMBERS OF ALLOWED CARTS, VACANCIES, WHEN AND
HOW TO SWITCH FROM ONE PART TO ANOTHER, BASED ON THE ADVISED NEUTRON SQUARE
BASIC GEOMETRY AND ITS INHERITED ELLIPTIC/wave EQUATIONS.
Now detailed in BaseGroupMassNumbers.
Type (the Division
Algorithm deduced mod
operator: A mod B = subtract
B from A until rest is <B:
— if A<0 and B>0 the operation is locked on rest = A<0)
Rest(-A;2) = -A
-A mod
2 = -A
THE APPENDING WINDOWS 7 OPERATING SYSTEM CALCULATOR HAS A mod OPERATOR:
• it works
perfectly the same as the concordant division algorithm,
• but not in
Microsoft EXCEL —
• and not its
imitating OpenOfficeCalc.
a STRICT
NUMERICAL solution in OpenOfficeCalc looks like this (detailed
ex: MproblemSolved):
INT(√1+nHe/|nHe|) → +nHe = 0 ¦ –nHe = 1
THE SQUARE ROOT OPERATOR ALWAYS TAKES THE SQUARE DIAGONAL,
AND THE INTEGER OF THE SQUARE DIAGONAL IS ALWAYS THE SQUARE SIDE
1
INTEGER(ROOT(1+nHe/ABS(nHe))
[HELTAL (ROT
(1+nHe/ABS(nHe))]
→ +nHe = 0
¦ –nHe = 1
(1 –
[HELTAL (ROT
(1+nHe/ABS(nHe))])
→ +nHe = 1
¦ –nHe = 0
MsWORKS — Borland’sDelphi
Rest(nHe;2)
=
continue ..
SWEDISH REFLECTED — steady growing
interest in preserving biodiversity and caring for universal animal rights: It
is such a joy;
Tala om att
stänga vägen för mänsklighetens naturliga naturvetenskapliga utveckling:
• För ändamålet och tillfället: Att få
bedriva affärer — som tillvarons
allra högsta enda
mål
• av DRIFT. Inte plan. Inte avsiktligt.
— Enbart genom ett girighetsfall. Egobegär. Dess euforiska
domäner leder sinnet in på vägar som täcker för allt annat.
Mänskligheten
får inte tillgång till Resurserna — copyrightägda juridiskt köpta kärringar
skyddar hela verksamheten: lag. Den mänskliga historiens i särklass värsta
huggsexa.
— H. Ugga:
— Vad har du för
utbildning?
— The foundation of freedom, justice and peace in the world.
— DE BLIR SKITFÖRBANNADE. NÄR DERAS VÄRDERINGSLUSTA AVTÄCKS
FÖR DEN SKITIGA AVGRUND DEN ÄR, UPPRESER SIG PÖBELN OCH KRÄVER KORSFÄSTELSE.
— I sinnlig bemärkelse. Inte ett enda vettigt ord. Inget människorättsligt
igenkännande. Inte ett pip. Tvärt om. Hugg och slag, lydnad
och bestraffning. Ingen utbildning.
— OCH MAN UNDRAR: VILKEN
SKITAKTIGHET ÄR DET EGENTLIGEN SOM STÅR ALLRA HÖGST?
— Är det att avliva en obekväm, rent fysiskt? Eller är det att
häva ur sig sådana elakheter mot offret, rent verbalt, att offret begår
självmord —— enbart för att slippa vidare se utseendet på kräket?
Inte ett ord MänniskoRätt. Inte ett pip. ”.. varje individ och varje organ i samhället ..”.
Befolkningen undanhålls kunskaperna.
Det är inget fel på intelligensen. Problemet: låg moralisk
nivå: Lydnad och bestraffning. Ingen utbildning. Ingen undervisning.
Urlåg ordning — allt avgörs genom flest
gillapoäng. Ingen resonerande, relaterande, beskrivande och förklarande
förmåga. Lydnad och bestraffning.
[HELTAL(ROT
(1+nHe/ABS(nHe))] *nHe + (1 – [HELTAL(ROT
(1+nHe/ABS(nHe))])*Rest(nHe;2)
MicrosoftEXCEL — OpenOfficeCALC.
IF(nHe<0 ; nHe ; Rest(nHe;2))
MicrosoftEXCEL — OpenOfficeCALC.
— Microsoft — after MsWORKS 2008 — is definitely not
mathematically educated. No way. But, please: do disclaim: show examples.
— Breaking, Hacking, Chopping.
— ”Restart your computer, or we will do it for you”. Free
open jurisdictional certified experimentation enterprise on humanity mind
manipulation and decision.
NuklidTab4A2023.ods TableA From NuklidTab4.wks 2008 — finally solved for OpenOffice and Microsoft EXCEL
ALL STABLE ISOTOPES UP TO ATOMIC NUMBER 27 — max
mass number 60 —
the NeutronSquare horizontal square side
scale
The population is stranded on a culture where it is only
allowed to use 1% of the brains capacity ..
Microsoft
Enterprise alternative (C++)
HighIQ solutions?
Who in this universe would understand a
STRUCTURE of
IF A>B AND C .. while K AND not
D in .. where IF L=E AND ..
instead of
THE MUCH MORE VIEWABLE AND GRASPABLE TYPE
λ = INT[1–(abs[Z0/03–1] – [Z0/03–1])/2] ........................................ Zλ = .. ,51,41,31,20,10,00,–10,–20, ..
ζ1 = INT[1–(abs[Z0/01–1] – [Z0/01–1])/2]
........................................ Zζ = .. ,51,41,31,21,11,00,–10,–20, ..
γ10 = INT[1–(abs[Z0/10–1] – [Z0/10–1])/2] ......................................... Zγ = .. ,111,101,90,80, .. ,20,10,00, ..
Meaning:
— A regular rail-yard system with (many
trains, ranks, of) whole numbers and defined flipping and flopping relays has
(supreme) a more explaining overviewing power than any other (here) known
method.
See all the details in
Related physics meets
established physics on atomic nuclear presentations
HIGHEST SCORED MASS DEFECT CONTEST
THE PREFERENCES ARE SWITCHED true
1432 against untrue 3241 unless a more thorough explanation exists:
— What we know: atomic mass and its properties cannot be
described solely from the point of view of nuclear properties. The electron
mass must be included.
HIGHEST SCORED MASS DEFECT — COMPARING ATOMIC WITH NUCLEAR: ATOMIC IS THE CLEAR WINNER
— The Table1 exerpt below compares mass defect values in electron masses (1me = 0.511 MeV) between
atomic mass defect mD(atomic) = (1 – U/Amn)/me and
nuclear mass defect mD(nuclear) = (Amn – Zv – U)/me ¦ FM1975 s124sp2ö ”ΔM = ZMp + NMn – Mnuc” where
U = m(ATOMexperimentallyMeasured)/u(m[6C12/12]=1.66033 t27 KG) ¦ HOP1967 ¦ BerkeleyNational 2003 ¦ Nist/Codata 2005
A mass number
Z atomic number — nuclear charge, atom’s electron mass charge
mn = 1.0086652000u
me = 0.0005485982u
v = mn – m(1H1=1.007825200u) = 0.00084000000000u
mD(atomic) = (1 – U/Amn)/me
apparently COMPARING NUCLEAR
completely unknown in modern corridors
Never18: HIGHESTmD
Never @18
Highest mass
defect atoms — COMPARING
ESTABLISHED AND RELATED ATOMIC AND NUCLEAR PHYSICS
18Sep2023 — enhanced and more detailed version from Nov2007
— exact values depends on chosen constants
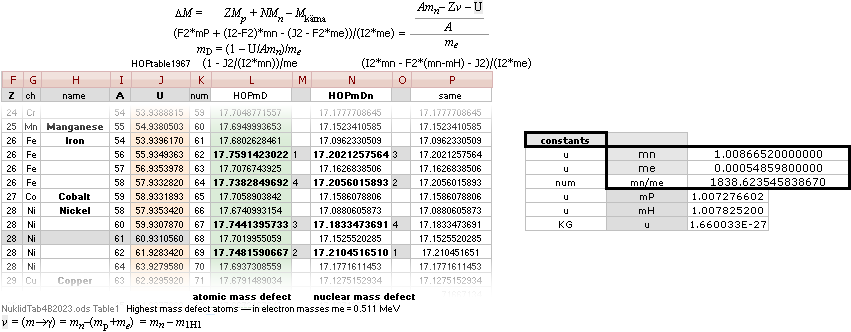
NuklidTab4B2023.ods Table1
The difference:
Nuclear mass defect (Amn
– Zv – U)/me uses A Z U. Atomic mass
defect (1 – U/Amn)/me uses only A U.
THE ATOMIC MASS DEFECT SOLUTION
IN NEUTRON SQUARE HAS ONLY ONE BASIC EllipticTrigonometricHyperbolic
form: mD = 6 + (1/5)√
60² — (60 — [ A–K]²)/E see NSsolutions
Compare The rZ
results from Atomic Nucleus in related physics:
♦ atomic/nuclear
mass and nuclear radius have no space metric connection to nuclear charge —
neutron basics: the neutron decays to an atom; the
neutron has no (significant) nuclear charge.
♦ mass — gravitation — and charge — heat and light, electricity and magnetism — does not connect.
COMPARE The Related Physics Deduction of The Electric Charge Q
—— no direct mass connection: Q² =
(m/R)(A/dT); Q²/m = A/RdT; m = Q²RdT/A = mA/RdT × RdT/A = m. No light E=hf connection.
♦ light does not connect
kinetics [Light’s
Liberty Clause].
See explaining details in Proton Radius and Atomic
Nucleus.
U = m(atom — from
mass spectroscopic experimental measures)/u —
older (1960+) ”atomic weight”, present (2023) ”relative
atomic mass”.
In UniverseHistory: just U you [ = Real Weighable Mass/u].
u = 1.66033
t27 KG (the Dalton unit), = m(6C12)/12
— different sources in different epochs have different u.
COMPARE WIKIPEDIA, (Atomic mass):
” At the peak of binding energy, nickel-62 is the most tightly bound nucleus (per nucleon), followed by iron-58 and iron-56.[19]”,
WIKIPEDIA
Nuclear binding energy curve (19Sep2023)
The present science community is not aware of any other
preference than the already present established:
— ”Atomic mass defect” is not a scientific community established term — no such article in Wikipedia.
———————————————
ORIGINAL Nov2007: IronTOP ¦ ComparingMAC ¦ Formula ¦ Atomic
Nucleus — Jul2023¦ ProtonRADIUS
CompHmD: HIGHESTmD
Further comparing examples and
sections
COMPARE again THE CONCEPTUAL DESCRIPTION on atomic mass defect, the table above:
IRON 26Fe5617.759 is the strongest built atom that needed the highest working energy waste to build, correspondingly the hardest most energetically demanding atom to restore on its original bricks, followed by Nickel 28Ni6217.748, 28Ni6017.744 and again Iron 26Fe5817.738.
The population is apparently a
related victim of a too meager developed terminology in atomic and nuclear
physics. We
find the exact same order of business in comparing from the more regular
equations
atomic (AmN – U)/A
nuclear (AmN – U – Zv)/A
NuklidTab4C2023.ods TabellB A1+
Introducing ”Z” transfers values to nuclear mass defects — A LOWER ORDER OF STRONGEST most wasted mass energy work on its building BINDING ATOMIC ENERGY.
Iron it is.
See also (Sw) the Odd and Even Nuclide Groups — ending on IRON (from zero neutron quote, related), the (CAP) reason why all primary celestial bodies develop an iron core center.
See also further in
FAMQ ¦ FIBAPO ¦ DIFFERENCE BETWEEN ATOMIC AND NUCLEAR ¦ Clarifying the U equation
unless already familiar — related.
:
Introducing ”Z” in the calculations only
decreases the maximum atomic strength.
— »Well .. I give a shit about atomic. I’m just interested in nuclear».
Further exemplifying the
different concepts
THE COMPARING NUCLEAR
— DIFFERENCE BETWEEN ATOMIC AND NUCLEAR
The comparing nuclear diagrams examples (internally calculated comparing values from experimentally measured atomic masses) further underlines the hazard and adventure in — generalizing and favoring — the idea and concept of ”nuclear binding energy”:
(FIBAPO): Unaware of the Neutron
Square solutions,
it was impossible to foresee any a coming conflict. Humanity was so involved in
the industrial progress from the technical revolution. And it just evolved and
grew. The wealth of the world ..
As developed during the 1900s, present (2023) academic quarters still uses a concept of type ”binding energy potential to the number of nucleons” (HOPweizQuote1967 below) as connected to our complex describing ”a good approximation to the true masses”.
’good
approximation to true masses’
That is the whole story point in this presentation:
the accurateness in presenting the (2003) discovered NS natural neutral atomic
mass quantities. And the tools, the theories and their difficulties (compared)
involved to reach a related explanation.
The present scientific
community
The ’binding energy per nucleon’ concept relates to the classic modern academic idea of the atomic nucleus as enveloping freely inner (spinning) existent neutrons and protons. As commonly termed nucleons, these relates to a The present established explanation of the atomic nucleus and its physics.
The terminology ”Binding energy per nucleon” (Wikipedia, Atomic mass¦Nuclear binding energy¦Nuclear binding energy curve, Sep2023) is also the present (2023) only apparent (freely available) concept in our highly beloved Earth science community when discussing and presenting associated atomic mass subjects on a theoretical (calculable) basis.
In the atomic mass defect preference, table above HIGHESTmD,
” At the peak of binding energy, nickel-62 is the most tightly bound nucleus (per nucleon), followed by iron-58 and iron-56.[19]”,
WIKIPEDIA Nuclear binding energy curve (19Sep2023)
— The present science community is not aware of any other
preference than the already present established.
— You know, the one type »We know everything»-standard. MustBuyBook.
”.. most tightly bound nucleus ..”
— »When we have removed all the Zv electrons from the atom, the most tight bound atomic nucleus is ..».
— There is no atomic nuclear physics understandable relatable reason in the statement.
Never18 IN RELATED ATOMIC NUCLEAR PHYSICS:
the leading maximum atomic tight
binding energy top is reserved for iron-56 followed by
nickel-62 and nickel-60: In concern of a general presentation of an
Atomic species and its property, other preferences than an Atomic one will
cause confusion. Nuclear binding energy always exposes a lower quantitative
value.
DIFFERENCE BETWEEN ATOMIC AND NUCLEAR
¦ Clarifying the U equation
— THE »Take» in modern corridors is
understandable — other top rated atomic individuals — WHERE academic theory
lacks terminology:
” The page ”Atomic mass defect” does not exist”.
Wikipedia 9Oct2023.
A search on ”mass defect” on the other hand is redirected in
Wikipedia to the article on
Nuclear binding energy.
nuclear mass defect
atomic mass defect MDa = AmN – U ¦ U = mATOM/u ¦ u = m(6C12)/6 = 1 Dalton
nuclear mass defect MDn = AmN – U – Zv ¦ v = nino = mN – (mP + me) = mN – m1H1 (= 0.00084u)
MDn – Zv = MDa MDn = MDa + Zv.
v: the work mass energy waste that built
the first atom — 1H1 Hydrogen — from the fundamental atom: The Neutron [ FAMQ
]
THE DIFFERENCE BETWEEN ATOMIC AND NUCLEAR (binding energy, mass defect) can
apparently not be clearly explained and related without understanding the
concept of atomic mass defect (Clarifying the U
equation):
The choice of »explaining everything by
nuclear preferences» is the only one available
in established corridors. With the introduction of the Neutron Square paragon solutions (NSsolutions) in related
physics (TNED),
the present scientific idea is (partly, or perhaps completely) overrun: no
direct comparing is possible (other than: two different property domains).
———————————————
HIGHESTmD ¦ Comp ¦ ComparingNuclear
ComparingNuclear: Comp — Compiled 9Oct2023 ¦ Difference
between atomic and nuclear — illustrated on experimentally measured
values
In modern standard academic quarters — atomic and nuclear
physics — there is only one single basic preference: experimentation.
Neutron Square Solutions has no such. It is a PLATE: neutral atomic mass defect
values for comparison. No electric or magnetic experimentation details or other
involved — other than the computer (Windows 3.1, Paintbrush) on which the
discovery was made (2003) in studying the (HOP1967) atomic mass
tables. The reader might himself lure out the rest, as also this author is an
equal standard tourist on the page.
PURPOSE (NSsolutions):
COMPARING ATOMIC MASS DETERMINATION
Main Weizäcker liquid drop
model equation — nuclear mass defect type
B(Z,N ¦ MeV) = 15.75A
– 0.711000Z2A–1/3 – 17.8A2/3
– 23.7(A – 2Z)2/A ±¦0: 11.18A–1/2 + Zv ¦ WeizCalifornia 2023 NSdeDIA13
B(Z,N ¦ MeV) = 14.00A – 0.61Z(Z–1)A–1/3 – 14.0A2/3 – 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 adjust. ¦ HOP1967 NSdeDIA10
General attitude:
ANY COMPARING CONTEST
between nuclear mass defect and atomic mass defect
on any quest of precise ATOMIC masses
WILL BE OUT OF THE QUESTION
Statement:
On observation of established claims
connecting atomic mass to nuclear binding energy
———————————————————————————————————
There is no available cosmic way, path, or other known universal opening to calculate a precision atomic mass from nuclear mass defect — No way.
Oct2023:
But it seems like the scientific community is claiming exactly that, unless we here in UH are victims of even worse misunderstandings.
— This author would be happy if that also had been explained from the beginning.
— Well then, NeutronSquare Solutions suggests — in clarifying the issue:
♦ Let us compare: Weiz1:1967¦1975 — Weiz2: WeizCalifornia2023 — Weiz3 u=m(6C12)/12 ¦ CONOR.
NSdeDIA5: CoNu ¦ DIFFERENCE BETWEEN ATOMIC AND NUCLEAR
INTERNAL EXPERIMENTAL MEASURED VALUES WITH DIFFERENCE
BETWEEN ATOMIC AND NUCLEAR
NuklidTab4C2023.ods Tabell1 Col. V W X BM
Proof:
WEIZSÄCKER EQUATION DETAILS AND
PARAMETRIC CONSTANTS SOURCES:
HOP 1967
— HANDBOOK OF PHYSICS, E. U.
Condon, McGraw-Hill 1967, section 9, p.9—8
— HOPweizQuote
The Swedish FOCUS MATERIEN 1975 s125sp2n
GENERAL ATOMIC MASSES DATA — concurrent [ except marginal on
last decimals on later measures ]
HOP1967 BerkeleyNATIONAL2003 NIST/CoDATA2005+
NSdeDIA8: — THE ![]() 1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION — NSde5
1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION — NSde5
— See the present Wikipedia ¦ California University Weizsäcker
versions in WeCALu
Revisting
FIRST (2003+) OBSERVED DIFFERENCES BETWEEN
EXPERIMENTAL AND TNED-ACADEMIC(MAC)
HOPweizXP.ods Tabell1 Col. F G O
———————————————
Neutron Square Solutions — NSsolutions ¦ The
Atomic Nucleus ¦ THE DIAGRAM DIFFERENCES — NSdeDIA9 ¦ THE here used WEIZÄCKER
EQUATION PROGRAM — WeizPRO
1. The precision aspect (N) is already occupied by Neutron Square solutions — directly beginning from the neutron (Neutron Square Fundamentals — BasicCHART ¦ FIBAPO).
2. The established claims connecting atomic mass with nuclear binding energy uses the (foremost) so called semi-empirical mass formula (WIKIPEDIA, 15Oct2023 — WikiWEIZ) — most known as the Weizsäcker liquid drop model atomic nuclear equation. As so recognized (WikiWEIZ quote):
B(Z,N ¦ MeV) = 14A – 0.61Z(Z–1)A–1/3
– 14A2/3
– 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 ¦ HOP1967 example
” The semi-empirical mass formula therefore provides a good fit to heavier nuclei, and a poor fit to very light nuclei, especially 4He.”,
WIKIPEDIA,
Semi-empirical mass formula (as quoted 15Oct2023).
The poor part is visually obvious as further clarified below in NSdeDIA9. But the ”good fit” part — where is it except on the intersecting regions @6Carbon12 and @10Neon20¦21¦22? Wikipedia (15Oct2023) gives no quantitative example.
However further ahead here, we will come back to that.
NSdeDIA9: The NSdeDIA8 comparing DIFFERENCES: — THE ![]() 1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION
1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION
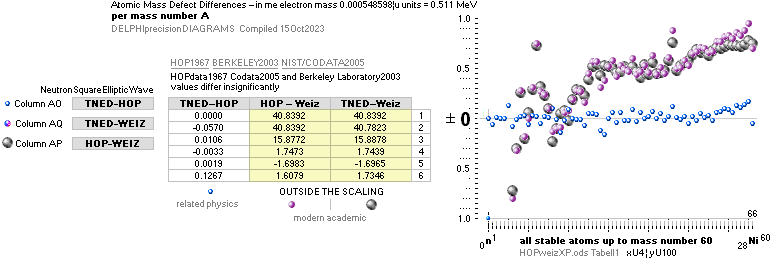
HOPweizXP.ods Tabell1 Col. AO AP AQ
A possible lead (» .. they don’t know it ..»):
See Wikipedia quote in Not
represented — Clarifying the U equation.
— They the general scientific community apparently don’t know It.
Say it: THAT IS COMPLETELY WRONG — Further [» .. they don’t
know it .. »] Clarifying Examples will follow.
NSdeDIA10: The
Weizäcker PROGRAM — THE ![]() 1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION
1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION
the parameters — different sources — vary depending on
application — and epoch —
here assumed [ as proven ] not really deviating much from the
presented:
B(Z,N ¦ MeV) = Zv+ 14A – 0.61Z(Z–1)A–1/3 – 14A2/3 – 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 ¦ HOP1967
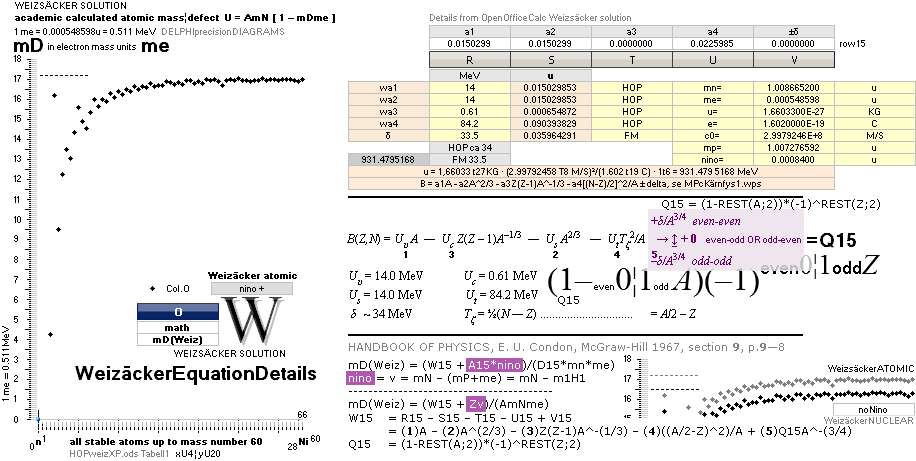
HOPweizXP.ods Tabell1 Col.O
B(Z,N ¦ MeV) = 14A – 0.61Z(Z–1)A–1/3 – 14A2/3 – 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 ¦ HOP1967
B(Z,N ¦ MeV) = 14A – 0.61Z(Z–1)A–1/3 – 14A2/3 – 84.2(A – 2Z)2/A ±¦0: 33.5A–3/4 ¦ FM 1975 ¦ N–Z=A–Z–Z=A–2Z
MeV → m(ATOM): e(MeV)T6/c02 ;
MeV → U = mATOM/u(Dalton):
MeV → U: e(MeV)T6/c02u ¦ U = mATOM/u ¦ 1u = m(6C12)/12 = 1.66033 t27 KG
———————————————
HOPweizQuote1967 ¦ WeizsäckerCALC ¦ WeizsäckerEquationDETAILS ¦ WeizäckerEVENoddCriteria — the Q15 solution
WhatZv: Zv
What’sUp?
— The thing is this:
atomic mass defect MDa = AmN – U ¦ U = mATOM/u ¦ u = m(6C12)/6 = 1 Dalton
nuclear mass defect MDn = AmN – U – Zv ¦ v = nino = mN – (mP + me) = mN – m1H1 (= 0.00084u)
The (HOP1967) Weizsäcker »nuclear binding enery»
”binding energy potential to the number of nucleons” equation
B(Z,N ¦ MeV) = 14A – 0.61Z(Z–1)A–1/3 – 14A2/3 – 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 ¦ HOP1967
is apparently — related — of the type nuclear mass defect (nuclear binding energy).
♦ But NSdeDIA10 lower right of the illustration
• the Weizsäcker equation formula as such has no inside referring »–Zv»

HOPweizXP.ods Tabell1 Col.O — Weizsäcker ATOMIC-mD HasA+Nino=Zv ¦ Col. AN — Weizsäcker NUCLEAR-mD HasNoNino=Zv
so that the end station exhibits this building:
the experimental
nuclear matches the Weizsäcker atomic on its + 1Zv
or/and
the experimental
atomic matches the Weizsäcker atomic on its
+ 2Zv
right below:
♦ The HOP1967 ¦ FM1975 Weizsäcker equation formula as such has no inside referring term »–Zv I am A Nuclear concept» or factor:explicitly declaring it is a nuclear mass defect expression.: it lies below the experimental nuclear MDa = AmN – U.
For it to reach
the experimental nuclear for comparison, an extra additional +Zv
must be added:
NSdeDIA11: NSdeDIA10
Comparing results on THE ![]() 1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION
1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION
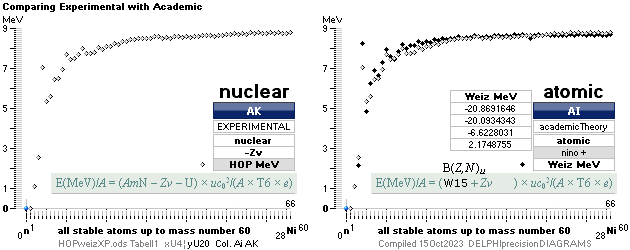
HOPweizXP.ods Tabell1
B(Z,N ¦ MeV) = 14A – 0.61Z(Z–1)A–1/3 – 14A2/3 – 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 + 1Zv ¦ nuclear-atomic HOP1967
So: In »fixing a most close match» between the two agents, the Weizsäcker form must either add one Zv to its original to reach the experimental NUCLEAR level — right part above:
— »ExperimentalNuclearMatchesWeizsäckerAtomic»;
Or add one extra Zv, in all 2Zv, to reach the fairly good matching experimental ATOMIC level:
— »ExperimentalAtomicMatchesWeizsäckerDoubleNuclear», right below:
NSdeDIA12: NSdeDIA11
Comparing results on THE ![]() 1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION
1967 ¦ 1975 COMPARING WEIZSÄCKER VERSION
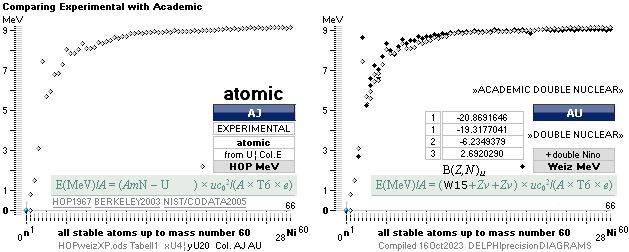
HOPweizXP.ods Tabell1
B(Z,N ¦ MeV) = 14A
– 0.61Z(Z–1)A–1/3 – 14A2/3 –
84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 + 2Zv
¦ atomic-DoubleNuclear
Besides these minor points:
♦ The Weizsäcker general solution is a horrible nightmare for the first light atoms — always outside the vertical scale.
• The basic idea (charged liquid drop model) apparently does not
apply at all to the atomic nucleus as a corresponding fair model. Not even
close to (FIBAPO).
Or as already stated (ComparingNuclear — proof continued):
There is no available cosmic way, path, or other
known universal opening to calculate precision atomic mass from nuclear
mass defect — No way.
— Why? Because it apparently already is occupied by the Neutron
Square solution complex: geometric, trigonometric, elliptic,
hyperbolic ¦ CompCALu2023.
— On the other hand, as deduced: The only difference between atomic mass defect MDa and nuclear mass defect MDn hangs on one single factor term: Zv. If no objective argument is visible, the simple way to make adjustments between the two is by adding or removing the factor Zv term.
See the comparing Weizsäcker diagrams in ComparingNUCLEAR.
See Wikipedia quote in Not
represented.
Missing vocabulary: It seems like
the modern academic idea has pushed Nuclear and Atomic into one more or less
arbitrary useful Hollywood popular computer modeling galactic houseboat,
traveling around the neighborhood, collecting near space data for further
popular academic enlightenments.
ANY COMPARING CONTEST
between nuclear mass defect and atomic mass defect on any quest
of precise ATOMIC masses
WILL BE OUT OF THE QUESTION
The present versions (16Oct2023) as collected @Internet on available sources:
There is (Oct2023) a COOKIES BLOCKED
SCIENTIFIC FORUM
physics.stackexchange.com ¦ COMPARISON BETWEEN
BETHE-WEIZSÄCKER-MODEL FOR NUCLEAR BINDING ENERGY AND MEASUREMENTS ¦ posted 2017
mentioning »The Weizsäcker mass
equation syndrom».
— At first, in the Internet younger
days, it was open for any Earth inhabitant to read and fetch inspiration from,
now sadly partly blocked. A Weizsäcker equation diagram draft has been
programmaticly exposedby a person — very extensive and visually educative —
however not fully completed (or so recognized):
— The point to show was: ”the claimed
precision does not seem to fit”.
The contributor refers to a German
source (”Handbuch der Physik”) claiming ”the model should match the data with an error of 1 percent for large A”.
— The right end part above in NSdeDIA12 A=60 ends on a 4% deviated match (assumed
to decrease further with growing A — stable atoms exemplified).
— So: where precision?
Try Comp2023.
Further explained an related below in NewWeiz2023,
NewWeiz2023: NSdeDIA12
THE NEWER WEIZSÄCKER SOLUTION VALUES
— The 1967¦1975 Zv
issue removed ..
The development of electronics and the advent of powerful computers and new measuring techniques on all science branches — especially from the 1980s (Intel’s first microprocessor 8080) — made way for the Weizsäcker equation to reach new precision levels: chasing the nuclear origins behind the experimental results. We know that and also in part how by comparing the early (1967¦1975 — Comp) use of the Weizsäcker form with the present: 2023 Wikipedia sourcing articles, California university Comp2023 and other free document contributors.
The WeCALu (2023) diagrams below in NSdeDIA13 are the same as in NSdeDIA8 the 1967¦1975 version type, but now with modified constants, and some apparently significant and important changes on the actual algebraic structure (a clear adoptive change as seen in the comparing):
CHANGES:
Former [ 1967¦1975 ] most tight Weizsäcker match HOP1967
NSdeDIA10 :
B(Z,N ¦ MeV) = 14A – 0.61Z(Z–1)A–1/3 – 14A2/3 – 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 + 1Zv ¦ nuclear-atomic
B(Z,N ¦ MeV) = 14A
– 0.61Z(Z–1)A–1/3 – 14A2/3 –
84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 + 2Zv
¦ atomic-DoubleNuclear
The California University version
B(Z,N ¦ MeV) = 15.75A
– 17.8A2/3 – 0.711Z2A–1/3
– 23.7(A – 2Z)2/A ±¦0: 11.18A–1/2 ¦ WeizCalifornia
has automatically adjusted the earlier 1967¦1975 literature
»nuclear-atomic»
level issue
Col.O: The additional 2Zv 1967¦1975 NSdeDIA8 Weizsäcker NSdeDIA11 ”double nuclear” has disappeared completely, leaving only (the
now corrected) single +Zv correction. But it is still not so mentioned in the
actual PDF source (California University):
♦ IF the WeizCalifornia expression is taken
as is
B(Z,N ¦ MeV) = 14A
– 0.61Z(Z–1)A–1/3 – 14A2/3 –
84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4
— no included Zv = nino —
the level of the right below Weizsäcker diagram receives the NSdeDIA14 same flaw as the earlier
exposed — in this new costume a corresponding »ExperimentalAtomic matches
Academic Nuclear»:
The California Match:
B(Z,N ¦ MeV) = 15.75A
– 17.8A2/3 – 0.711Z2A–1/3
– 23.7(A – 2Z)2/A ±¦0: 11.18A–1/2 + Zv
¦ atomic-atomic
is the diagram below right, and its experimental matching
below left.
— The change in parameters between the old and new epoch has
— apparently — automatically removed one Zv from the old standard, »at the same
time» raised that lower level to the former included Zv end result. With so
only one »still integrated» Zv, the Weizsäcker has reached »atomic mass defect
standard».
By striving to approach
the experimental results, the theoretical reasoning is forced to approach ![]() the Neutron Square Solution complex:
neutral atoms. No involving electric or magnetic. No charge math. Guaranteed
intrinsically electric clean.
the Neutron Square Solution complex:
neutral atoms. No involving electric or magnetic. No charge math. Guaranteed
intrinsically electric clean.
THE READ CURVE BELOW [ latest 2020 results — BAGMAN
] IS THOUGH NOT OPTIONAL— WE STILL HAVE MUCH WORK TO DO ON DERIVING THE MORE
PRECISE ELLIPTIC ATOMIC MASS SOLUTIONS, ATOM BY ATOM.
See also further comparison in
from
ComparingNuclear ¦ NewWeiz2023
NSdeDIA13: WeCALu: NSdeDIA8
B(Z,N ¦ MeV) = 15.75A – 17.8A2/3 – 0.711Z2A–1/3 – 23.7(A – 2Z)2/A ±¦0: 11.18A–1/2 ¦ WeizCalifornia
See the below left Weizsäcker
diffference to the Experimental and TNED in Comp2023.
THE ![]() 2023 COMPARING California University VERSION
¦ and other associated similar
2023 COMPARING California University VERSION
¦ and other associated similar
HOPweizXPa.ods Tabell1
NSdeDIA14: WhatZv
B(Z,N ¦ MeV) → mD¦me: e(MeV)T6/mec02u — Why the 18 scale? Never 18 — Captial18e.
— But the point is still: our included Zv
— the left match above — is NOT the California University formula result, the
here representative present 2023 Weizsäcker form. That one
B(Z,N ¦ MeV)
= 15.75A – 17.8A2/3 – 0.711Z2A–1/3
– 23.7(A – 2Z)2/A ±¦0: 11.18A–1/2 ¦ WeizCalifornia
lies lower [ Col.AN ]:
THE PURE WEIZSÄCKER EQUATION RESULTS REFLECT ..
— Just compare on the HOP nuclear alternative ..
— Do not lose the objective:
— Aim: atomic masses comparison (NS).
Not nuclear.
HOPweizXPa.ods Tabell1 Col.AN
It is mildly spoken confusing
— So: What’sUp?
There is apparently something
the institution does not tell the visitor .. if the visitor did not forgot his
shoes at home .. or the teacher has some holes in his universe ..
WeizCalifornia: ThePureWeizsäcker ¦ WeCALu
THE found 13Oct2023 UNIVERSITY OF CALIFORNIA free PDF has a detailed presented comparing picture with given and explained Weizsäcker parametric vales — not easy at all to find @Internet as a comparing source:
———————————————————————————————————————
E(binding, MeV) = B(Z,N ¦ MeV):
![]()
B(Z,N ¦ MeV) = 15.75A – 17.8A2/3 – 0.711Z2A–1/3 – 23.7(A – 2Z)2/A ±¦0: 11.18A–1/2
NUCLEAR
PHYSICS ¦ University of California ¦ Phys 239 Quantitative Physics Lecture 18
NUCLEAR
PHYSICS free PDF, p2.Eq.(1)
MeV → m(ATOM): e(MeV)T6/c02 ;
MeV → U = mATOM/u(Dalton):
MeV → U: e(MeV)T6/c02u ¦ U = mATOM/u ¦ 1u = m(6C12)/12 = 1.66033 t27 KG
B(Z,N ¦ MeV) = 14A
– 14A2/3 – 0.61Z(Z–1)A–1/3 –
84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 ¦ HOP1967 NSdeDIA10 ¦ CalOrder
B(Z,N ¦ MeV) = 14A – 0.61Z(Z–1)A–1/3 – 14A2/3 – 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 ¦ HOP1967 NSdeDIA10
B(Z,N ¦ MeV) = 14A
– 0.61Z(Z–1)A–1/3 – 14A2/3 –
84.2(A – 2Z)2/A ±¦0: 33.5A–3/4 ¦ FM 1975 ¦ N–Z=A–Z–Z=A–2Z
B(Z,N ¦ MeV) = aVA
– aCZ(Z–1)A–1/3 – aSA2/3
– aA(N – Z)2/A + δ(N,Z)A–3/4
¦ Wikipedia, Semi-empirical mass formula,
15Oct2023,
no specified composition example: ”.. can again be derived by modeling ..”, ”.. not easily explained ..”.
— So: WHAT IS THE DIFFERENCE?
Again:
See Wikipedia quote in Not
represented.
Is the present scientific
community in a deep lack of explaining terms, or have we missed something?
THE DIAGRAMMATIC EXPERIMENTALLY
measured DIFFERENCE ![]() BETWEEN
BETWEEN
atomic MASS DEFECT ![]() AND
AND
nuclear
MASS DEFECT ![]() as equated
as equated
atomic mass defect MDa = AmN – U ¦ U = mATOM/u ¦ u = m(6C12)/6 = 1 Dalton
nuclear mass defect MDn = AmN – U – Zv ¦ v = nino = mN – (mP + me) = mN – m1H1 (= 0.00084u)
is as illustrated below from
experimentally measured atomic mass values
— but no established literature seems
to describe or explain the terminology.
NSdeDIA7: AtomDiffNuc: FAMQ
The mass defect concept
DIFFERENCE BETWEEN ATOMIC AND NUCLEAR
The chart below is a partial extraction from the more generally extensive atomic nuclide chart (free available tables @Internet).
![]() ORANGE dots: MDa = AmM – U
....... atomic mass defects
ORANGE dots: MDa = AmM – U
....... atomic mass defects
![]() SkyBLUE dots: MDn = AmM – U –
Zv nuclear mass
defects
SkyBLUE dots: MDn = AmM – U –
Zv nuclear mass
defects
part of the
atomic chart exemplifying the concepts
JUST CLARIFYING THE MANY SEEMINGLY
CONFUSING basic and elementary TERMS AND CONCEPTS IN ATOMIC AND NUCLEAR
PHYSICS
A mass number [ FAMQ],
mN = 1.0086652u neutron
mass, U = mATOM/u = atomic weight, u = m(6C12)/12 = 1.66033 t27 KG = 1 Dalton, Z atomic number = nuclear charge, atom’s number of electron
masses
DIAGRAM: Atomic mass defect, Nuclear mass defect — often
”nuclear binding energy” in modern corridors — and their difference by quantity
Data source: part of 1967 HOP table on
atomic masses — with minor end decimal differences, same values as in the
tables from BERKELEY2003
and NIST/Codata2005 — the latter
have more isotopic posts
The atomic mass
defect
![]() demands more WASTE energy input to become U than the available
demands more WASTE energy input to become U than the available
![]() minimum nuclear mass
defect. The difference is:
minimum nuclear mass
defect. The difference is:
![]() = Zv = Z(mN — mH1) ¦ mH1 = 1.00782519u difference
between atomic and nuclear
= Zv = Z(mN — mH1) ¦ mH1 = 1.00782519u difference
between atomic and nuclear
THE MORE WASTE MASS-ENERGY INPUT FOR ASSEMBLING THE END
PRODUCT, THE HARDER TO REGAIN: the ”binding energy” concept:
— »My motivation to withhold information for you, is
stronger than your attempt to get such info». »Binding energy».
All atoms are built from a number (A) of (FAMQ) fundamental
atomic mass quanta called a NEUTRON (Chadwick 1932)
with mass mN. AtomOrigin = A × mN. The neutron itself so also contains the first atom
(1H1, Hydrogen). It shows on the (ca 12 to14 minutes) neutron decay in credit
of its natural atomic unstable property, ending on the first stable atom,
hydrogen mH: proton nucleus (mP) + 1 electron mass (me).
RELATED MDa:
All relations of the form
(AmN — U)/A or
(AmN — U)/AmN or
(AmN — U)/AMnme
belong to the same basic general ATOMIC (total) mass
defect concept (MDa).
It is a value for the mass working
energy needed to complete the entire atomic assembly. Entirely, or an averaged
over the original number of A masses mN — or further expressed in electron
masses (me).
RELATED MDn:
All relations of the form
(AmN — U — Zv)/A or
(AmN — U — Zv)/AmN or
(AmN — U — Zv)/AMnme
belong to the NUCLEAR mass defect
concept (MDn) — often ”binding energy per nucleon” in established literature.
It exposes a lower quantity than MDa —
another type of mathematics — never including the entire atomic energy
(building) concept:
v
= mN — mH1 ¦ the actual neutron decaying mass working energy waste in raising the building of the
hydrogen atom:
— The atom’s nuclear charge Z balances
the actual atom’s electron mass envelope.
The atomic mass defect (MDa)
![]() AmN — U demands more WASTE energy input to become U than (MDn)
AmN — U demands more WASTE energy input to become U than (MDn)
![]() AmN — U – Zv the minimum nuclear mass defect. Difference is:
AmN — U – Zv the minimum nuclear mass defect. Difference is:
![]() = Zv = Z(mN
— mH1) ¦ mH1 = 1.00782519u ¦ v = 0.00084u
= Zv = Z(mN
— mH1) ¦ mH1 = 1.00782519u ¦ v = 0.00084u
THE MORE WASTE MASS-ENERGY INPUT FOR ASSEMBLING THE END
PRODUCT, THE HARDER TO REGAIN: the ”binding energy” concept:
— »My motivation to withhold information for you, is
stronger than your attempt to get such info». »Binding energy».
— »What’s the difference?».
— »Love me or leave me». [ ITERATION PRINCIPLE ].
AtomDiffNuc ¦ ComparingNuclear
COMPARE THE FOLLOWING:
Wikipedia, Nuclear binding energy (Oct2023)
” Nuclear binding energy in experimental
physics is the minimum energy that is
required to disassemble the nucleus of an atom into its constituent protons and
neutrons, known collectively as nucleons.”.
THE REFERRED TABLE IN THE Wikipedia ARTICLE
REFERS ONLY SINGLE ATOMIC MASSES, THERE IS NO CALCULATING COMPARING EXAMPLE
between two different masses on releasing an example-neutron ALIKE THE BELOW NSdeDIA6:
NSdeDIA6: WikiQuote
EXPOSING THE BINDING ENERGY CONCEPT exemplified
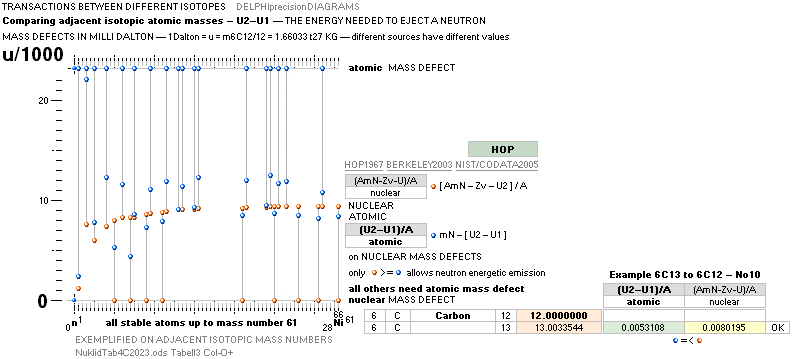
Explain:
IN The ![]() 18 cases of the
exemplified extracted 27
18 cases of the
exemplified extracted 27
the minimum
energy that is required to disassemble the nucleus of an atom into
releasing energetically the equivalent Planck
energy E=hf=mcr/t=mc² one neutron from a
heavier atom U2 resulting in a lighter isotopic atom U1
is defined by the atomic mass defect
(AmN — U)/A.
Only in ![]() 9 cases of the
exemplified extracted 27
9 cases of the
exemplified extracted 27
the minimum
energy that is required to disassemble the nucleus of an atom into
releasing one neutron
from a heavier atom U2 resulting in a lighter atom U1
is sufficiently provided
by the lower existing nuclear mass defect
(AmN — U — Zv)/A, or ”the nuclear binding energy” quantity.
Compare:
Wikipedia, Nuclear binding energy (Oct2023)
” Nuclear
binding energy in experimental physics is the
minimum energy that is required to disassemble the nucleus of an atom
into its constituent protons and neutrons, known collectively as nucleons.”.
APPARENTLY SOMETIMES.
Yes.
But definitely not
always.
As the exemplifying
extracted experimental values NSdeDIA6 diagram shows.
See also comparing diagram
AtomDiffNuc:
Atomic lies always above
nuclear in total maximum BINDING energy.
What means ”binding”?
THE MORE WASTE MASS-ENERGY INPUT FOR ASSEMBLING THE END
PRODUCT, THE HARDER TO REGAIN: the ”binding energy” concept:
— »My motivation to withhold information for you, is
stronger than your attempt to get such info». »Binding energy».
— »What’s the difference?».
— The natural »Love
me or leave me» principle. [ ITERATION PRINCIPLE ¦ IterationTheorem ]:
— »I might get looser on you if you show me some kindness
..».
COMPARE (vulgarized):
— I give a shit about more or less, if it is the least, it covers any quantity over.
— Yes. OK. So if you are asked of an investor How much money do you have in the bank? .. you would expect a reasonable response in answering .. At least one dollar : it fucking covers any quantity over. Yes? I didn’t think so. We have to get educated — related answers only.
THE VOCABULARY HAS MISSING PARTS
We cannot blame or critizise the Wikipedia authors or other
sources on this noted and observed issue. Because, the problem apparently is
this: A central explaining term is missing — in the established teaching and
educating system;
See Wikipedia quote in Not
represented.
— All these revealing
facts have only the Neutron Square solutions as a source. And that source was never observed in modern
quarters.
— So that the reader will
understand, know and be able to relate that these ”new statements” are in no
way any mumbo-jambo hocus-pocus gravel.
Examples: InternetExample
Further EXAMPLIFYING THE CONFUSING CONCEPTS —
that must be understood by thorough examplifying and comparing examples:
A direct quote from the
same Wikipedia article Nuclear binding energy just underlines »the confusion of
tongues»:
Provision:
The Wikipedia article does not say
Mnuc = U – Zme ¦ nucleus
gravitating mass
+
electrons gravitating
mass = atom
gravitating mass:
Mnuc = U – Zme ¦ nucleus
gravitating mass
= atom
gravitating mass — electrons gravitating mass.
”total
binding energy”
” If we assume the reference nucleon has the
mass of a neutron (so that all ”total” binding energies calculated are maximal) we could define the total binding energy as the difference from
the mass of the nucleus, and the mass
of a collection of A free neutrons.
In other words it would be
(Z + N )mn — mnuclide.
The ”total binding energy
per nucleon” would be this value divided by A.”,
WIKIPEDIA, Nuclear binding energy, Example values
deduced from experimentally measured atom
masses (12Oct2023).
(Z
+ N )mn — mnuclide ”the total
binding energy”, Wikipedia article says
—
No.
(Z
+ N )mn — matom the total
binding energy, related physics and mathematics says
(Oh please .. Someone has to cut the oxygen from
this rebellious intruder .. questioning everything ..)
Explain:
See Wikipedia quote in Not
represented.
(Z
+ N )mn — mnuclide ; the
Wikipedia quote [ Z + N = A ] ;
AmN — (U — Zme) is not a ”total binding energy” ¦ WikiWEIZnuclearMass
quoted comparison:
SHORT: The Wikipedia article postulates a more demanding
expression for ”the mass of an atomic nucleus” :
We test that part with a related provision of a gravitationally
related nuclear mass concept, U – Zme, and find that the Wikipedia stated
expression is verified. Then, the continuing:
AmN — (U — 0.00) is. RelatedNUCLEAR
MDa = AmN — U atomic mass defect = total atomic FULLY RELATED
binding energy:
MDn =
AmN — U—Zv nuclear
mass defect = nuclear binding energy — ClarifyingTheUequation
— always less
APPARENTLY related unless we
lost some vital parts of our mother ship when entering the on ramp IT IS THE GENERAL ATOMIC MASS DEFECT —
fundamental atomic mass Quantum binding power MDa — that ON ITS MINIMUM determines weather or not a neutron release
is possible, our 18 U2 minus U1 examples NSdeDIA6.
— So ..IF the article had written ”(Z + N )mn
— matom” we would be safe:
We would be in a perfect agreement with the
results:
total binding energy relates to all the wasted working
mass-energy needed to reach the end atomic mass (atomic weight U = m/u) U station.
— If other
understatements hold, confusions arise unless explicitly specified.
♦
Atomic — in most cases, exemplified NSdeDIA6 the least binding energy to allow a
neutron emission.
♦
Not nuclear.
Related
physics TNED ¦ NScredit
The
atomic nucleus U = m(ATOM)/u has no inner np-constituents. No way. It has a
fundamental np nuclear structure. No np particles. We only use
these n (neutron) and p (proton) in addressing (AZchart) their fundamental nuclear structural type morphological
properties. Not any kind or nature of »their particle forms». We talk: ±e
structure.
Nuclear
charge (Z) in related physics has no volumetric substance. No way. Z is a well
relatable ±e nuclear surface electric displacement (with a corresponding
nuclear magnetic moment), stretching (rZ) no further out from the
nuclear center than the gravity radius of the nuclear space extension (r).
See the finally collected (already existent before our eyes) experimental proofs in The Atomic Nucleus.
— What is this author trying to say?
Provably :
THE present academic IDEA OF NUCLEAR MASS
DEFECT
”BINDING ENERGY PER NUCLEON”
COMPROMISES ANY THEORETICAL CALCULATION OF ATOMIC MASSES
FOR COMPARING on EXPERIMENTALLY MEASURED.
— Why? CompareQm:
The NSdeDIA5 Diagram exhibits the relatively huge
corruption between atomic and nuclear:
Because
gravitation the
fundamental atomic nucleus/atom The NEUTRON has no particle constituents inside of it:
Gravitation is
not a particle.
Its fundamental
physical Form, the basic atomic nucleus, the Neutron h=mcr is NOT a
particle, has no particle constituents. See Nuclear
STRUCTURE. It is proving that the Atomic Nucleus experimental results,
no way sir, can be successfully modeled by any macroscopic physical idea type uniformly charged liquid drops as in the Weizsäcker equations.
And it — gravitation, the atomic nucleus, the neutron — definitely cannot be
compressed: it is already standing on a zero [Spin and Moment, Background].
— There is nothing wrong with
the experiments, the experimental results. No way. These are accurate.
— Problem is The Aces behind
the keyboards pushing in new parameters to make the design more adopted to
Hollywood Experimental Enterprise. Experiments are fine. The Aces have been
collected into a constantly leaking Boat, left in the middle of The Pacific.
— How do we explain the later (WeizCalifornia ¦ CompCALu2023) more precise Weizsäcker
nuclear binding energy solutions precision with the experimental results?
♦
Always CHASING THE EXPERIMENTAL WITH FILLING IN
COMPUTER MODELING:
• Because that is the only provisional
preference the present scientific community has: it has no NS.
• And so, seen from the NS-position: The
Weizsäcker rank mathematics solution or any other liquid drop nucleonic model, no matter the
computing power fails the target :
neutral (TNED) atomic masses. NS solutions. Weizsäcker solutions and associated is guaranteed locked out, no matter the experimental precision
or the computing power.
— Or, if these statements
will fail: NS solutions has no value, not at all. Dodge that one, anyone who
can:
FIRST BASIC PROOF — Geometric
———————————————
ComparingTable ¦ WeizsäckerEquationDetails
Any comparison
between NS solutions and any theoretical
mathematical idea of calculating atomic masses from an idea of uniformly
charged liquid drops directly becomes out of the
question. The present academic scientific community idea of the atomic nucleus
as inhabited by ”nucleons inside the nucleus” is apparently (very, and deeply,
and fundamentally — related) cosmologically and universally definitely
incorrect.
— But it is the
only one available in a referring past science history, all beginning from
around 1900. We grew up with it. Everywhere. Merits.
— »Good dog».
Unaware of the
Neutron Square solutions, it was impossible to foresee the coming conflict. On
the other hand: Without that science history development, this (or any other existing,
what we know) author would definitely
never had come upon the Neutron Square discovery (2003 — from studying the
experimental values on atomic masses). So .. the established unawareness made way for the discovery — while the
unaware was collecting the answers: the imperative mass tables. The
instrumental lead.
— See further
from HIGHESTmD.
NuclearBindingEnergy ¦ ComparingNuclear
NSconditions: FIBAPO — first proof
NEUTRON SQUARE CONDITIONS (NScredit)
Related physics and mathematics — Neutron Square solutions — NSsolutions
———————————————————————————————
The atomic mass defect (mD)
mathematics is founded on the Neutron
Square and its deduced general elliptic equations (A
K E). It
generates — exposes — unique neutral as in experimentally independent
atomic mass calculated values. As compared to the experimentally tabled
results, the NS values apparently have close kinship (Actual
differences):
♦ The NS solutions have no dependency on the physical property of nuclear charge Z (DeducingTHErZ).
Modern academic scientific
community atomic and nuclear physics
———————————————————————————————
The nuclear mass defect traditional modern academic science theory [WeizCalifornia2023] uses different modeled macroscopic concepts (uniformly Z charged liquid drops) to describe the variations in atomic masses (A Z).
♦ Present academic solutions use of nuclear charge (Z):
— The (materialistic) idea is that the atomic nucleus consists of sub atomic inner freely spinning proton and neutron particles (A=n+p = n+Z): Z reflects determining significance on the nuclear size (and its mass).
Summing:
———————————————————————————————
♦ In Comp and ComparingNuclear (HOP167¦FM1975 Weizsäcker solution) it is readily apparent that the present academic idea of sphericity, drop modeling and uniformly distributed charge does not reflect the natural morphology of the atomic nucleus and its physical properties. That is explicitly obvious on the basic first light atoms. As a representative of explaining nature (science), we had expected something more alike the compared NS solutions results: tight, right from the beginning (FIBAPO).
See the basic (HOP1967¦FM1975) comparing examples in
NSsolutions ¦ FIBAPO ¦ EXPERIMENTALconfirmations ¦ CosmicMATHrevelation ¦ HIGHESTmD ¦ Comp ¦ ComparingNuclear
NSsolutions: INTRODUCED 2003 in UH —
NSconditions ¦ NeutronSquareFundamentals ¦ CosmicMATHrevelation ¦ AboutTheMETHOD ¦ Geometric
NEUTRON SQUARE SOLUTIONS
See the (related) basics in how the Planck constant (h=mcr) connects to (i.e., defines — verifies) the neutron square solutions, connecting the (related physics) Hydrogen Spectrum to a consistent derivation of the neutronic atom’s space basic metrics:
The radius of the proton and the neutron. Never included in modern quarters.
NEUTRAL ATOMS — charge-experimentally independent
electric and magnetic
experimentally independent atomic mass defect values
The Neutron Square Solutions complex
contains
trigonometric,
direct geometric,
vertical elliptic,
wave, horizontal
elliptic, and hyperbolic solutions to
NEUTRAL atomic mass defects mD. From mD a direct transfer
U
= AmN(1 – mDme) gives the atomic mass (U=m/u) in Dalton units
(u = mC12/12) for
direct experimental comparison (NScredit).
NEUTRON SQUARE FUNDAMENTALS
———————————————
TRIGONOMETRIC — basic¦ Trigonometric — geometry¦ Geometric ¦ Wave ¦ VerticalELLIPTIC ¦ Hyperbolic — basic ¦ HYPERBOLIC — detailed — Principle ¦ HorizontalELLIPTIC — SameAonDifferentU? ¦
What the reader should know,
first ..
Neutron Square solutions:
U
= AmN(1 — mDme): atomic mass m/u = U = [older] atomic
weight [
newer ] ”relative atomic mass”
A
mass number, mN 1.0086652
me 0.000548598 neutron electron mass in Dalton u=1.66033 t27 KG units = mC12/12.
mD atomic mass defect NEUTRON SQUARE
SOLUTIONS in electron masses, 1me = 0.511 MeV = 0.000548598u.
Nuclear Mass related physics NSsolutions
mNUC = U — Zme: Z nuclear charge as atomic number as the
atom’s electron mass charge
has no
connection to nuclear charge
in NSsolutions, related physics — AtomicNucleus
¦ NScredit
:
ELECTRIC CHARGE divergence AND GRAVITATIONAL MASS
convergence ARE TWO DIFFERENT PHYSICAL PROPERTIES — Never18.
In MAC mNUC is associated with nuclear charge: mNUC = ZmP + (A–Z=N)mN – B,
B nuclear/nucleon binding energy between the idea of the nucleus as
composed of inner freely spinning Z mP and N mN particles:
all established
academic experimental data are collected, ordered and compiled in accordance
with that program.
IN NS SOLUTIONS, IT HAS NO CONNECTION, as proved in AtomicNucleus:
all Angeli2004 collected experimental data.
The NS solution is EXPERIMENTALLY INDEPENDENT: neutral atoms
no charge dependency — NScredit.
— Either it is. Or it isn’t.
It is our job to clarify which it is.
SO, WE ONLY HAVE TO FIND ONE SINGLE ITTY BITTY THE SMALLEST TINIEST PROOF, whatever:
— Either it is, or it isn’t.
It is not about a contest. It is
about knowledge of the world we are born to: science. No consensus. Just pure
true natural knowledge — what can be proven, related, explained and understood,
inclusively. Or
not at all.
” Nowadays, several mass measurements are conducted with fully or almost fully ionized atoms. In such cases, a correction must be made for the total binding energy of all the removed electrons Be(Z).”,
” Unfortunately, the precision of the calculated Be(Z) values is not well established, since this quantity (approximately 760 keV for 92U) cannot be easily measured.”,
THE
AME2020 ATOMIC MASS EVALUATION, Wang et al., Chinese physics C 2021 ¦ free
PDF, p1
The Wang table has specified uncertainties: 1H1(1.007825 03190) → 0.00001 µu
THE DISPERSING BINDING ENERGY COMPROMISING AND
CORRUPTING NATURE ¦ see ComparingNuclear
— The Neutron Square atomic
mass defects have revealed the details in this complex — completely unknown in
modern quarters. Electric and magnetic features are imperative in
experimentation on atomic and nuclear matters. Yes.. But — as proven (ReHofstadter1956)
through NS
— nuclear charge and nuclear extension — volume, radius — does NOT connect. No
way. Charge and mass have no common physical ground, although integrated in the
atom (TheQ).
So — rhetorically: how do modern academic experimentation rinse the results
from such »experimental infection»?
Test Answer: »The PhD does not
understand the question». Not one person inside present scientific community
really knows what nuclear and atomic physics in reality is all about (NScredit).
The community just jumped on The Train, and followed the lead to the cafeteria.
” Modern mass-spectroscopic measurements employ the ”mass doublet” technique. One determines the differences in mass between ions of the same mass number having slightly different masses. Because there is only a small mass difference between the two kinds of ions, the instrument dispersion need not be known as accurately as would be the case if the masses differ widely ..”,
HANDBOOK OF PHYSICS, E. U. Condon, McGraw-Hill 1967, Nuclear physics p.9—64 col.2b
The HOP table has specified uncertainties: 1H1(1.007825 19) → 0.08 µu ¦
See ExperimentalErrors and ISOBARIC COMPARISONS.
PROVISIONS
THERMALLY INDEPENDENT ¦
ZERO GRAVITATIONAL POTENTIAL c0 environment — TNED K-cell physics : zero
gravitational redshift
Neutron Square
Fundamentals — one Unequivocal
atomic mass for one specific mass number A : — no. BACKGROUND:
The bare clean
fact ComparingIsobaric
that both the experimentally measured and the NS solutions UnequivocalAmD same A have
values lying VERY
close in U values:
CHECK OUT THE GENERAL very small
experimentally tabled DIFFERENCES in electron masses me BETWEEN stable ISOTOPIC PARTS FROM DIFFERENT ATOMIC FAMILIES [
isobaric]:
NuklidTab4B2023.ods
Tabell1 Col.Y+
— At most: 0.086 me; At least: 0.001 me — isobaric occasions for all
stable isotopes from 1H1 to 83Bi209. HOWEVER:
— THE MDa CONCLUSION on
Neutron Square solutions STATES and proves by principle THAT
THESE NARROW DIFFERENCES no
longer FAVORS A FURTHER AND DEEPER NS INSPECTION
ON THE isobaric EXPERIMENTAL RESULTS
THE NEUTRON SQUARE SOLUTIONS
— UNDER WHICH PHYSICAL CONDITIONS?
♦ apparently not EXACTLY the established scientific community experimental ones for starters:
WE — APPARENTLY — MUST ASSUME THE CORRESPONDING NS CONDITIONS
1. • no gravitational potential (ideal mass center) — highest possible divergence = max c0 =2.99792458 T8 M/S space (NSUunit)
2. • no heat — no specific [ heat ] energy fluxes in that g-free ideal dark space: 0°K — different regions have different conditions
3. ♦ experimentally gravitationally c0
dependent — where light propagation c in space is maximized, same as the
natural divergence constant c0 ¦ K-cell mass center;
we have no other
independent — GEOMETRIC
— neutral maximized atomic mass quantity to refer to.
4. ♦ HEAT charge electricity and magnetism IS NOT MASS (NScredit) —
and the NS solution neutral atomic mass complex is therefore, or can so therefore be understood as, absolutely independent of thermal regions.
The geometric neutral atomic NS provisions complex apparently just compare — or states — the actual gravitational conditions with those from any possible theoretical statement. It is our job to secure a certified interpretation of such comparisons, on whatever scale that relates to a scientific explanation.
See further precise examples from
Comparing Normalized (CONOR).
Universal example: h = mcr
Planck constant h = mcr — conservation of angular momentum (the neutron mNc0rN) should be equally determinable — independent of experimentally measuring region.
Caring for LGD-basics
(the TNED deduced universal K-cell mass center
gravitational redshift, modern academy’s ”dark energy” related to ”extra expansion”,
in related physics a standing still with corresponding Doppler red shift
instrumental readouts), the two factors mN and c can have inverse proportionality without
breaking the conservation of angular momentum.
Where (significant) gravitational redshift affects electric and
magnetic experimental measurements, the locally lower divergence c
then demands a proportionally higher resulting quantity mN
if h is to remain a universal constant.
As the experimentalists however — the absolute metrics — uses not the local c
divergence, but the universal (absolute metric) c=c0 quantity in all experimentation, the actual
higher mN value will reflect an experimental
position in an actual such c=c0 region. In other words: with a slightly received higher mass value for
mN than the actual where c=c0. Again: the true c=c0 region mass mN value will be slightly lower.
As suggested in NSUnit — universal atomic mass unit u
= m(6C12)/12 = 1 Dalton —
given a (c0 experimental laboratory based) measured electron mass me,
a comparing normalized (CONOR) neutron mas mN = 1/(1 – 15.6me) will — with no
exceptions — be less than that specific laboratory’s correspondingly measured
neutron mass (mNc).
By Wikipedia (finding U=A exemplified)
reported standard values, example:
mew = 0.0005485799u Wikipedia original electron mass
mNw
= 1/(1 – 15.6mew) = 1.0086317155u; u-normalized
mN(W) = 1.00866491588u
Wikipedia
original
mNw/mN(W)
= 0.9999670848 — Planck
constant local c-divergence u-correction:
h = mcr = universally
constant, no exception; h/r = independent universally constant = mc
— lower local c = consequential
but not real mass higher m, LGD-basics
The farther away from the ideal g-center (TNED K-cell related
physics only), the greater the difference. At the universal c-rim
(c approaches zero) the experimental mN
mass would display an experimental approach to infinity — on the laboratory’s c0 use;
— »In for a penny, in for a pound».
LGDbasics: RELATED
PHYSICS ONLY — Provisions ¦ NSUnit
———————————————
FOREMOST PRACTICAL APPLICATION: GPS-example ¦ CWON from CAP — concentric atomic
production ¦ light’s gravitational dependency ¦ NScredit
While light’s gravitational dependency
(LGD) is recognized in modern corridors experimentally as the so called
Mössbauer effect, its corresponding reversed spouse from the gravitational
center with increasing enveloping mass — universal gravitational redshift,
related physics — is, more or less, heavily denied (See modern academics ”dark
energy”, Sw., Mörk
energi).
In related physics and mathematics
(Sw., K-cell
heat physics) the universally cosmic LGD explaining core is a
central pulsating K-cell (half period: 336Gy).
It is maintained, and mathematically
explained, by a gravitational energy balance between a central and a
surrounding mass complex. This complex is governed by LGD light’s gravitational
dependency (General cosmic state law). And as such,
it is founded on the universal Planck constant h=mcr and the energy law: energy cannot be
created, nor be destroyed (Physics first principle
¦ ExperimentalConfirmations),
only converted.
The quest of the origin of mass
(modern academy 1800+ postulated a
limited existential mass, perhaps by reasons more relating to the Bible’s ”In
the beginning” literary interpretation, than the alive eye of a child — and it
has so become a mission for the academy to preserve that delusion)
has no logic or reasonable resolution,
because the origin of energy¦mass has none:
— Everywhere we look, there is always
more behind. Incontestably experimental fact.
(Modern academy 1800+ always had deep
existential problems).
It is all related mathematics.
See further exemplified in
(comparing normalized).
CONOR: comparing normalized — 25Oct2023
INTRODUCING EXAMPLE IN Provisions ¦ ComNorm
COMPARING NORMALIZED
NSUnit ¦ LGD-basics — CONNECTING CONTEXT — defining a universal
atomic mass unit u = m(6C12)/12 = 1 Dalton
———————————————————————————————————————
RELATED TNED MATHEMATICAL K-CELL´PHYSICS
The (2021) Andromeda
Test result on partly not easily nor precisely reliable data
however suggested that the TNED K-cell universal gravitational
center — where c=c0 resides — possibly would have this overviewing orientation:
The modern
academic consensus is that the measured Doppler values are proving a future
collision course between our Milky Way galaxy and the Andromeda galaxy.
However, related physics suggests, taking that Doppler shift on a gravitational
red shift scale, a mathematical complex apparently not known inside modern
corridors, the explanation is: No. Not at all. The galaxies are suggested to
have a very close standing still status, possibly with some very minor
rotational feature involved. They are not going to penetrate each others discs,
at any future — ever.
A gravitational Doppler red
shift suggested for our position a most
0.999633079 as corresponding to a local c-correction against the top divergence
c0 = 2.99792458 T8 M/S. As these data are more or less highly uncertain, a
certain possibility exists that — if at all — »the true» positional deviating
value would be some
”0.9999670848” — »WikipediaStandard»
Much closer to our position.
Believe it or not. Even though the different
epoch standard values (1960-1999 instrumental ¦ 2000+ data computerized) have
relatively large inside differences (the TNED
2018 IAU Sun photometric effect test revealed that both will not do, as far as
yet tested), in taking these different standards on the comparing Normalization
Neutron Square u-testing preference, they render practically the same end
station result:
mew = 0.0005485799u Wikipedia original electron mass
mNw
= 1/(1 – 15.6mew) = 1.0086317155u; u-normalized
mN(W) = 1.00866491588u
Wikipedia
original
mNw/mN(W)
= 0.9999670848 — Planck constant local c-divergence u-correction:
h = mcr = universally constant,
no exception; h/r = independent universally constant = mc
— lower local c =
consequential but not real mass higher m, LGD-basics
meh = 0.000548598u HOP1967
mNh
= 1/(1 – 15.6meh) = 1.0086320026u; u-normalized
mN(H) = 1.0086652u
HOP1967
mNh/mN(H)
= 0.9999670878 — Planck constant local c-divergence u-correction:
h = mcr = universally constant,
no exception; h/r = independent universally constant = mc
— lower local c =
consequential but not real mass higher m, LGD-basics
On testing this Neutron Square
Solutions suggestion
— our Earth Solar system experimentally
provision in the universe has a
0.99996708
true c0 diversion, covering (atomic, nuclear) mass measuring in a slight
deviation from universally true mass quantities
— the already given GPS-example will serve as a first
Testing Guard:
— Is the GPS system satellite signaling
technology mathematics results in any the slightest possible way, even mathematically
theoretically, affected on this NS suggested minor true c0 diversion?
Apparently: no. Not at all.
In our GPS test — proving the validity
of the TNED deduced results to be in accord with the present GPS technology and
its reported quantities — we had this compiled resulting table on all the used
and comparing variables (Deduced connections):
GPSexComp: CONOR
Deduced connections ¦ Compiled TNED GPS-test results — no
relativity theory involved
NOW we take the exact same table — with
only one single difference:
We replace the Laboratory Standard
Earth Academic c= c0 2.99792458 T8 M/S used in the equative solution, with the
u-NS-comparing-Normalized suggested
c = c0 × 0.99996708
c = 2.99 782 591.164946 M/S
Then we take the two tables, the
original GPS test table and the suggested cNSComparingNormalized, ANDING them
through each other picture-wise, looking for any deviation in figures. And this
is what appeared:
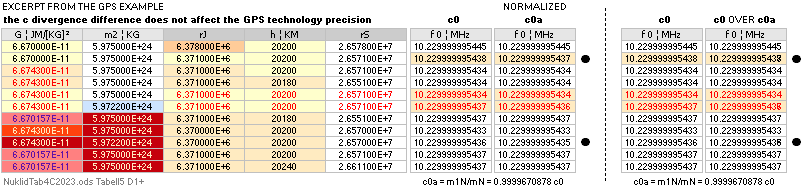
NuklidTab4C2023.ods Tabell5 D1+
No — what we know — significant change.
Same daily route.
No disturbance.
And that is apparently exactly what the Neutron Square Solutions u-defining provisions demand for a fair and correct universal comparison on neutral atomic masses m=Uu.
The above GPS oriented test proof, does not mean that the
above suggested universal c0 deviating possibility IS the explanation to the
Normalization level shift, as illustrated below. The effect of the mN
normalization level shift MIGHT — not known here — as well be
caused by a (mass-to-charge experimental) issue in measuring the experimentally
unaffected NS neutron mass preference (Normalization). The NS
solution suggest an error of 100(1/0.99996708 –1) = 0.00329%.
In any case it is
clear that the Weizsäcker general ”uniformed charged liquid drop model” has
nothing to do with the NS solution complex. The Weizsäcker model in the NS
light clearly appears as a sophisticated academic game in chasing
experimentally perfected computer animations: exactly zero explaining power. Or
the reverse: »the NS solution complex is a complete heap of ancient rocky
gravel crap». Please do share the revelations.
ComNorm: CONOR
Comparing differences in me:
THE GENERALIZED TNED NS SOLUTIONS from A=60 up to 83Bi209
HERE REPRESENTED BY THE GENERAL SIMPLE NS HYPERBOLC EQUATION The Gold Hyperbola [ Sw. orig.
10Oct2004 ]:
![]()

HOPweixXPa.ods ¦
NuklidTab4C2023.ods
General first
atomic chart part rough band Neutron Square solutions (NS)
deviation: 0.45 me = 0.00025u. The NS independent neutral atomic solutions
suggest that the experimental values have systematic — »variational» —
mass-to-charge issues locked on habituated computer modeling adopted to
match experimental results. The Normalization
as such have, apparently, only two available explanations. 1. The 0.99996708 c0
dislocation possibility, or: 2. A corresponding misapprehended interpretation
of an experimental evaluation of the neutron mass on a 0.00329% basis. Or, at
worst, a combination.
— Which it is,
any way, will be our job to find out. Or, perhaps the most academic imperative:
to get rid of this presentation, as it threatens modern academic ideas to
distinction: »so many cannot be wrong», and the like. Type: »you don’t have
enough amount of pals to get in here». HighSciIQ.
The first light atoms in the Weizsäcker
solutions lie far outside the vertical scale.
— No way the modern academic idea of
the atomic universe reflects a just and fair model of the atomic nucleus. Not
even on my best day.
— The »modern PhD aces» are apparently
more interested in chasing the experimental values by using computer modeling
parametric algorithms, than studying the basics of our universe. Say that :
that is completely wrong. Say it.
Weizsäcker solution is chasing the experimental
and the experimental is chasing the neutral — the NS
neutral atomic masses:
the experimental
cannot dispense with electric and magnetic field strengths:
The Neutron Square solutions has nothing of the kind.
NS solutions it is, suggesting:
Experimental deviations from true
atomic masses exist — possibly but not settled partly due to a less understood
universal laboratory position.
Dodge that one, anyone who can.
— We surrender immediately on any
related disclaimer.
Modern academic ideas of atomic and nuclear physics is not
just outclassed and overrun: It is buried.
But please, anyone: do disclaim on any related
argumentation.
We will surrender immediately.
EllipticEquation: Neutron Square solutions ¦ Geometric ¦ Vertical Elliptic
The neutron square and
THE ELLIPTIC EQUATION —
The ELLIPTIC
EQUATIONS ¦ NeutronSquareBasic for NeutronSquare
solutions — NSconditions ¦ Neutron Square Fundamentals
The
Neutron Square sophisticated paragon system — as deduced, departing from The
Neutron as Planck constant h = mcr
K = AREF (1 + mD’/6), see OFFSETfactor
positions the exothermal fusion path
ellipse
If mD’ relates to the Neutron, mD’ = 0, one receives K = A =
AREF
AREF = 6(1–E)
K = (1–E)(6 + mD’) ¦ E=0 → K=(6 + mD’) ¦ E=1 → K=0
Exothermal
fusion paths from lighter atoms with already determined mD as mD’ to heavier is
the foundation:
mDmethod: Geometric
Generally one must first realize a
thorough — related, deduced — evaluation for a certain atom and its nuclide in certifying
it is accountable as an exothermally valid formed atom. That is, the nuclear
fusion path (see FusionRING)
must ascertain a giving-out-energy process at its formation, the actual mD end value.
That evaluation gives basic data on the factors Aref and mD’ (nuclide
reference’s atomic mass defect). These are imperative for realizing the
calculation, leading to the end station mD value. All such calculations depart
from the BASIC NUCLIDES (FIBAPO), themselves, on the NeutronSquare geometry only: basic
values appear directly/geometrically in the Neutron
Square. See basic illustrated examples in Geometric,
unless already familiar. From these, all heavier atoms are formed.
UnequivocalAmD: mDmethod ¦ MDa
Atomic mass defects on Neutral
atoms — NeutronSquare solutions
mD = 6 ± (1/5)√ 602 – (60 – [A – K]/E)2
different A can have same mD:
U =
AmN(1 — mDme) ¦ :
same mD on different A give different atomic masses m=Uu.
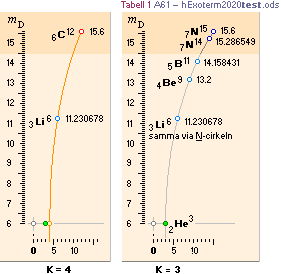 The 3Li6Group
The 3Li6Group
A = K + E(60 – √ 602 – [5(6 ± mD)]2)
different mD can have same A —— provided that
A =
K + Ek
; K = A — Ek
; E = (A — K)/k
A =
(1–E)(6 + mD’) + Ek
.. investigating the possible mathematical solutions may be
extensive ..
— What we know: As far as we can come from »all
combinations have been examined»
— here at present Sep2023 in UniverseHistory: We can only,
at present, state what has been found on the examined cases.
— More related arguments and precision
examples are needed before any regular general atomic chart will appear (all
stable atoms up to 83Bi209).
Provided an exothermal fusion path (FusionRing)
can be related and deduced, the above basic elliptic atomic mass defect
equation apparently allows different mD on same mass number A. Unless we can
prove that it does not exist, we cannot say.
♦ What we can say (AtomicNUCLEUS):
♦ Nuclear charge (Z) has no part in the NS solutions
atomic mass determinations.
♦ No Z dependency exist on atomic mass: mass and charge does not
connect in physics (NScredit).
Further resolution in MDa
(conclusion).
ISOBARIC ATOMS
The general NS solutions by the commonly compiled
elliptic and wave equations (EllipticWave)
show several different mD on same A :
♦ The NS calculated isobaric (same A) value differences on the light
chart part up to A=60 (see also IsobaricMEAN
¦ ComparingIsobaric
¦ ExperimentalErrors) are small: at
most 0.011 me, at least 0.001 me
(averaged minus actual), 1me = 0,00055u;
NuklidTab4B2023.ods TableB — all stable up
to A=60 :
A36:Z16,18
¦ A40:Z18,20 ¦ A48:Z20,22
¦ A50:Z22,23,24 ¦ A54:Z24,26.
Averaging these mD calculated differences, will render no visual
difference in the scales used here to present the resulting graphical and
diagrammatic picture. The differences decrease with increasing A.
However, where the solution is of the wave type[‡], there
should be a more precise regular elliptic value. At present (Sep2023) not all
occasions have been precisely analyzed.
Some parts in this (demanding — vast) analysis are exposed below.
The first iteration — before the
enhanced method — took three hours. Enhanced: ten minutes.
It is still deterrent.
CheckTabAElliptic: mDmethod
CHECKING NuklidTab4A2023.ods TabellA for
hExoElliptic
UP to A=60, all
stable:
16S36 17.311533460 HOP
16S36 17.367203106
NuklidTab4A2023.ods
TabellA wave
18Ar36 17.375553421
NuklidTab4A2023.ods TabellA wave
18Ar36 17.288717952 HOP
18Ar40 17.358932488 HOP
18Ar40 17.419527359 NuklidTab4A2023.ods TabellA wave
20Ca40 17.419527359 NuklidTab4A2023.ods TabellA wave
20Ca40 17.3137085 hExoterm.2020test.ods
Tabell1 17R elliptic
20Ca40 17.349684301 HOP
20Ca46 17.478866823 HOP
20Ca46 17.503586330
NuklidTab4A2023.ods TabellA wave
22Ti46 17.506348590
NuklidTab4A2023.ods TabellA wave
22Ti46 17.520408136 HOP
20Ca48 17.446656984 HOP
20Ca48 17.4795692 hExoterm.2020test.ods
Tabell1 173R elliptic
20Ca48 17.563718966
NuklidTab4A2023.ods TabellA wave
22Ti48 17.565741332
NuklidTab4A2023.ods TabellA wave
24Cr54 17.704877156 HOP
24Cr54 17.774214752 NuklidTab4A2023.ods TabellA wave
26Fe54 17.774214752 NuklidTab4A2023.ods TabellA wave
26Fe54 17.680262846 HOP
ExplicitElliptic: hExoterm.2020test.ods
Table1 — Excerpt, iterative results
Who did ever think of that in modern academic
corridors?
— »There is A neutral gauging ATOMIC NUCLEAR PHYSICS PARAGON the neutron square — explaining the detailed nature of nuclear and atomic physics (at c0).
— And mathematics (apparently very much).
— Concordant with experimental results.
— Including a further,
separate section, deduction of the periodic system of the elements from »simple
Kepler (momentum) Resonances». Basically »the same principle
mathematics» (nuclear matrix algorithm).
No.
Modern academic inducements had other ideas during its birth 1800+:
• The mission was — became — to Invent rather than Deduce.
As prosperous as that voyage went on, the merits
became established (experimental physics on the underlying theories):
university, high school, all kinds of established institutions: education. No
time for scrutinizing the basics. We adopt, or go bye-bye.
So .. this author chose to find another, more appropriate, tourist bus ..
See further in
AboutTheMETHOD: NSsolutions
The fundamental lead is the Neutron Square deduced Elliptic
Equations and their possibly deducible exothermal fusions
paths (VerticalElliptic), with some guidance
from the average simple wave equations (BaseGroupMassNumbers):
(the analysis
may be demanding .. always interesting .. only the end result will tell ..)
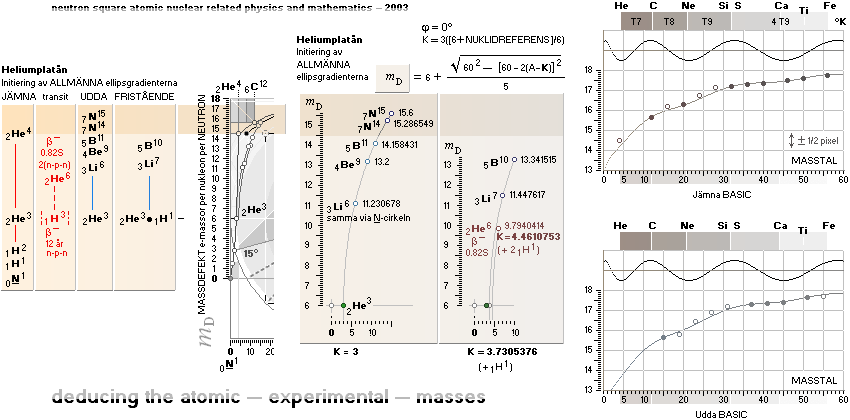
How nucleus
and its communicating electron mass cooperate in the atom — explaining the TNED neutron square atomic masses
deductions, the foundations ¦
basics
connecting the periodic system — excerpt
MPcKärnMatIIIb.wps 8Feb2008 — the nuclear n-p structural electric
displacements through resonances:
— See detailed in THE RELATED PHYSICS DEDUCTION OF THE PERIODIC SYSTEM THROUGH KEPLER RESONANCES.
central force dynamics
J = m(K=2A/T=2A
f =2n2f )KEPLER AREA resonance MOMENTUM, Jf –1=2n2
SWEDISH
EXCERPT:
J arbetar på elektronmassans
beståndsdelar; det bildas
stående, fasta vågmönster kring kärnan och som grundlägger, beskriver
och definierar hela atomens elektromekaniska jämviktssystem.
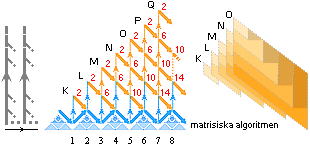
Kommunikationen
mellan kärna och hölje sker genom ovanstående avbildade kärnmatrisiska
algoritm, från kärnnivåerna (underst) till resonansnivåerna (överst), från lägre
till högre, och som ges av Keplermomenten. Den kärnmatrisiska algoritmen
definierar varje grundämne genom en bestämd uppsättning Z omgivande
elektronmassor som bildar ett helt resonanssystem. Den matrisiska algoritmens
successiva Z-bildningar definierar på det sättet grundämnenas periodiska
system enligt ordningen (i utdrag från utvecklingarna, typexempel, period
4)
… 2-8-8-1,
2-8-8-2, got it: 2-8-9-2, 2-8-10-2, … 2-8-18-2, I’m
on: 2-8-18-3, 2-8-18-4, …
1239410511612713814815816117218345670 .... 2-8-18-8 ...... period4
Vi använder
här 0 som en periodisk
avdelare genom en binär-hexal [oktal] avdelningsordning när vi räknar
successivt i matrisen, den allmänna 2-6-formen framgår inte direkt här utan
först när vi studerar upplösningen i Keplerresonanserna mera ingående.
ExperimentalErrors:
TheActualDifferencesHOP/TNED ¦ IsobaricMEAN ¦ CompCALu2023
A GOOD START IN DETERMINING THE
UNCERTAINTY STATUS OF THE NeitronSquare COMPLEX WOULD BE TO HAVE ACCESS TO THE
EXPERIMENTALLY STATED UNCERTAINTIES — and so we have through the early 1967
collected HOPtable
EXPERIMENTAL VALUES UNCERTAINTY —
IN NEUTRON
SQUARE ATOMIC MASS DEFECT
ELECTRON MASSES REFERENCE
— Comparing NS with Experimental needs a transformation from the HOP-table’s m/u=U (atomic weight) units to the electron mass unit in atomic mass defect (mD)
Atomic mass defect
equation (1 – U/Amn)/me
¦ U=m/u HOP-table values, A
mass number, mn me neutron electron mass in u = 1.66033 t27 KG selected value in
UH.
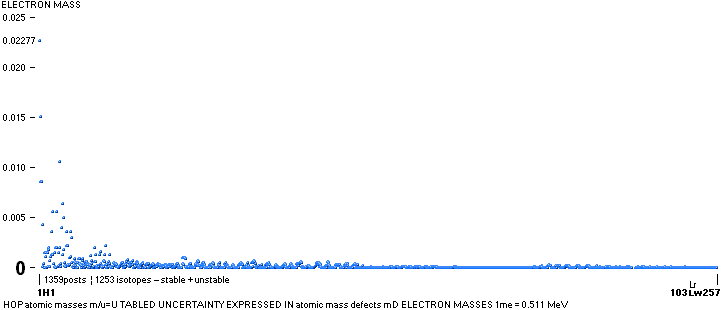
Atomic mass
uncertainty (positive value) in electron masses 1me = 0.511 MeV for the 1967 HOP TABLE 2.1. MASS TABLE
p.9—65+, HANDBOOK OF PHYSICS, McGraw-Hill 1967. Transfer
Equation explained below.
— Later table references (developed methods)
have (or claim so to have other (much more precise) error
specifications (WANG2021, see quoted description). The
above would represent the first scientific available during the 1900:s (ref. HOP
on spectroscopic method of atomic mass determination. The HOP-table
values source: from the 1964 ”least-squares reduction of data by Mattauch et
al.”).
— In itself, this
electron mass info has poor significance, unless compared to some atomic mass
calculating competitor, as the NS calculated values. Their difference here to
the experimentally measured, given in electron masses is related at first up to
mass number A=60 — here only the stable nuclei extracted (as also calculated
from the NS paragon).
HOPuncertainty: ExperimentalError
HOPtable uncertainty (ΔU) expressed (”Error in µu”) in a separate column for each atom as a positive integer value on
the t6:th (10–6) decimal of the m/u=U atomic weight (relative atomic mass) value
uncertainty = m(U) — lowest for the lightest (ΔU1H1 = 0.08), highest for the heaviest (ΔU103Lw257 = 1070).
EX:
(ΔU103Lw257=1070) × t6 = 0.001070
DifferenceMAX:
(U103Lw257=257.09894) – ΔU = 257.09787
By atomic mass defect mD —
corresponding tabled experimental uncertainty in electron
masses (positive value):
DIFFERENCE Expressed in atomic mass defect electron masses:
Atomic mass defect equation (1 – U/Amn)/me;
U(103Lw257) = 257.09894
V(103Lw257) = 257.09787
ErrorTransferElectronMass: HopUncertainty
mD(U) – mD(V) = (1 – U/Amn)/me
– (1 – V/Amn)/me ¦ A mass number, mn me
neutron electron mass in u:
= [(1 – U/Amn) – (1 – V/Amn)]/me
= [ 1 – U/Amn – 1 + V/Amn)]/me
= [ – U/Amn + V/Amn)]/me
= [ V/Amn – U/Amn)]/me
= [V – U]/Amnme
= [(U – ΔU) – U]/Amnme
= ΔU/Amnme
= (HOPtableError in µu)t6/Amnme
= (HOPtableError in µu)/(T6 · Amnme)
mDdiff =
(±)DIFFERENCE/uncertaintyExperimentalTabledHOP1967 in atomic mass defect
electron masses: = 0.0000016896 electron masses
uncertainty for
U(103Lw257) = 257.09894
mD = 14.9637550311 ¦ (±) 0.0000016896
Comp: Comp2023 ¦ HOP1967 —1975 EE¦ ComparingNuclear
TNED/MAC ¦ WeizSOURCES ¦ WeizCalifornia
COMPARING DIFFERENCES
MAC 1967¦1975 version:
NukTab4B2023.ods Tabell2
Comparing
precision in calculated atomic masses — ![]() TNED trough Neutron Square solutions and
TNED trough Neutron Square solutions and ![]() Weizsäcker
equations — with the 1967 HOP-table
presented experimentally measured — the horizontal zero line »exact value».
While the Weizsäcker solution shows vulgar differences on the first light
atoms, TNED/NS solutions appear readily »excellent» — especially on the first light atoms. See
in explicit the reason for the precision NS details from the Proton/NeutronRADIUS, unless already familiar (basic Planck constant math h=mcr).
Weizsäcker
equations — with the 1967 HOP-table
presented experimentally measured — the horizontal zero line »exact value».
While the Weizsäcker solution shows vulgar differences on the first light
atoms, TNED/NS solutions appear readily »excellent» — especially on the first light atoms. See
in explicit the reason for the precision NS details from the Proton/NeutronRADIUS, unless already familiar (basic Planck constant math h=mcr).
Comp2023: Comp — 1967¦1975 ¦ Orientation, see NewWeiz2023 ¦ CompCALu2023
MAC [ 2023 ] version:
HOPweizXPa.ods Tabell1 — WEIZSÄCKER EQUATION California University — NSsolutions — BaseGroupMassNumbers, general wave solution
up to mass number 60 — further calculated precision values are pending ..
The diagram above
reflects the new (Oct2023) enhanced Weizsäcker solution from a recently found
Weizsäcker California university (WeizCalifornia)
PDF document.
Related:
Difference to
above — exact comparing below should flip the above
TNED—HOP to HOP—TNED
——————————————————————————————————
The (13Oct2023) recently
found California university source (WeizCalifornia)
shows »a new Weizsäcker equation» form — never previously observed here in UH.
All (Comp) Weizsäcker data had so far (until now) been based on the HOP1967 ¦ FM1975 book sources on the Weizsäcker details.
The diagram above (Oct2023) exposes the
striking difference compared with the HOP1967 version difference (Comp). A prominent
advancement in precision has apparently been made.
See details in NewWeiz2023.
The
diagram below — now, finally, for the first time — shows the (California source
credited) overall TNED¦NSsolutions status on the comparing scale between TNED related physics and present Weizsäcker academic scientific
community. That is (for the first time) on the level of present academic
theoretical atomic and nuclear physics versus the neutral atomic Neutron Square
solution values.
See also the original (2008) Comparing
TNED/MAC, and the accompanying
»modern academic Weizsäcker nuclear-atomic» in NSdeDIA10: WhatZv.
CompCALu2023: NSdeDIA15: Comp2023
————————————————————————————————————
18Oct2023 — HOPweizXPa.ods Tabell1 :
Col.AP ¦ Col.F — Col.O HOP — WEIZ ![]() THE WEIZSÄCKER ATOMIC MASS EQUATION WeizCalifornia
THE WEIZSÄCKER ATOMIC MASS EQUATION WeizCalifornia
NuklidTab4C2023.ods Tabell2A Col.V-AA :
Col.AA ¦ Col.G — Col.Z HOP — TNED ![]() THE Neutron Square GENERAL Solutions
version [ discovered 2003 ] — the precision solution not yet available
THE Neutron Square GENERAL Solutions
version [ discovered 2003 ] — the precision solution not yet available
The TNED form explained by sources in NuklidTab4C2023.ods Tabell2A Col.V-AA optional— see further short description
below.
HOP EXPERIMENTALLY MEASURED COMPARING
PREFERENCE, Atomic masses table
For the moment, we leave
the comments to the reader.
Previous difference
comparison (the 1967¦1975 Weizsäcker version): Comp.
See details in NewWeiz2023
on the (13Oct2023 found) California version.
More will follow.
TNED — short:
A
mass number — from A=60: on the simple hyperbolic equation ¦ NuklidTab4C2023.ods
T2A Col.V-AA.
— When the hyperbolic part was first discovered as part of
the neutron square solutions, it was coined: The Gold Hyperbola [ Swedish original
10Oct2004 ].
Up to 2022 (TNEDa0.htm):
Neutron Square precision mD values (Elliptic Equation) demand a defined deduced fusion path from lighter to heavier — on the lighter already known mD values: Basic mD values ¦ FIBAPO (tool; exothermal nuclear reaction law, on confirming experimentally measured atomic masses/weights) Exothermal nuclear reaction law ¦ NUCLEAR REACTION LAW : it features a regular building, from bottom to top. Finding such an exothermal fusion path alerts elliptic candidates. An elliptic candidate binds the possible atomic products together on its path from lighter to heavier — but it must be so proven: Finding a suggested elliptic agent, it must satisfy (12-20 decimals, at least) a net difference (on known comparing agents) of type 0.00000000000000000000. Or at least 12 for starters. Then that solution has earned the status of preliminary Neutron Square precise solution. These solutions (at present) have only a (time consuming and demanding) manually iterative procedure to offer (TheExample), and far from all atoms have been tested.
The general Neutron Square solutions has, however, some more (fairly elegant, very) close simpler mathematical approximation provisions (geometric/trigonometric, wave and hyperbolic, see here most detailed the wave form in BaseGroupMassNumbers).
Up to latest 2020 it is those values with some precision elliptic that are presented here (Oct2023). Further work will attempt to make that picture more clear (provided the author will live say 514 years more, give or take ±12: the first manual iteration took over three hours)(and it must be checked, rechecked .. and double checked ..).
WEIZ — short:
The above (»Latest California World News») Weizsäcker expression now (Oct2023, WeizCalifornia) takes the lead in the continuing exciting story:
Chasing the fundamentals of our constitutional parts
— the atom- and nuclear physics atomic masses.
How they exist, and on what related precision.
The Weizsäcker equation form (from Weizsäcker 1935) is based on a regular charged liquid drop model (George Gamow) with thorough material physics properties, at least its original.
The Neutron Square solution has nothing of the kind. And that will apparently be our future challenge to explore the content of. For further.
Continue on
IsobaricMean: ExperimentalErrors
NSsolutions
Related physics TNED Neutron Square Atomic mass defect mD calculations —
entirely builds upon :
♦ comparing
on Experimentally tabled atomic masses from U=m/u atomic weight
tables — HOP1967, Berkeley2003, Nist-Codata2005
♦ no
nuclear charge Z dependency
— Z in NSsolutions is never used other than as an order number from lighter to
heavier atomic families.
EXPLAINING THE NEUTRON SQUARE SOLUTIONS
There
is no known experimental method to measure atomic masses without removing their
neutral property. Neutron Square solutions have perfectly neutral atoms, no Z
math.
Mass spectroscopic
methods excludes neutral — introduces parametric dependence — while their
physical nature still is not understood.
Comparing
ISOBARIC MEAN DIFFERENCE
———————————————
The striking (coherence NSdeDIA1
and) differences between the static-geometric Neutron Square solution values
(N) and the experimentally measured (E) apparently tugs (tows) some (heavy,
urgent need of) clarification.
— Unless, possibly: » .. that is a
natural .. spray in differences .. ».
Explaining
example:
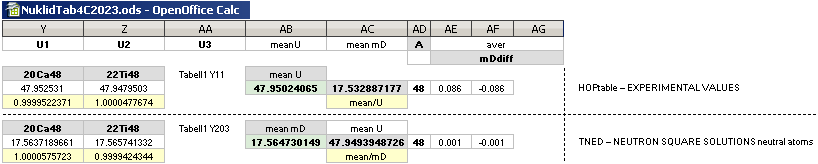
NuklidTab4C2023.ods Table1 Col.Y+
![]() The (Tabell1 Y11) experimentally measured (HOP1967) isobaric (A=48)
atomic mass defect mean value difference between 20Ca48 and 22Ti48 is: 0.086
electron masses.
The (Tabell1 Y11) experimentally measured (HOP1967) isobaric (A=48)
atomic mass defect mean value difference between 20Ca48 and 22Ti48 is: 0.086
electron masses.
![]() The (Tabell1 Y203) TNED Neutron Square solutions (A=48)
isobaric atomic mass defect
The (Tabell1 Y203) TNED Neutron Square solutions (A=48)
isobaric atomic mass defect
mean value difference between 20Ca48 and 22Ti48 is: 0.001 electron masses. Same object.
IF — as it is suggested — the neutral atomic Neutron
Square solution atomic mass complex is supreme[‡NScredit] to the experimentally charge
dependent atomic mass determination procedure, the relatively large
difference between the two isobaric agencies advices this explanation:
— Disturbing the neutral atom’s properties by introducing
charge dependent (mass spectroscopic electric and magnetic features) into the
mass determining procedure, adds a (difficult to determine, small) skewing
property — impossible to detect if only the experimental — not neutral atomic —
values have representation.
Further (or present) experimentation
should have capacity to resolve that issue.
Data source:HOP167
table —
all stable isobaric atoms 16S36 to 82Pb204
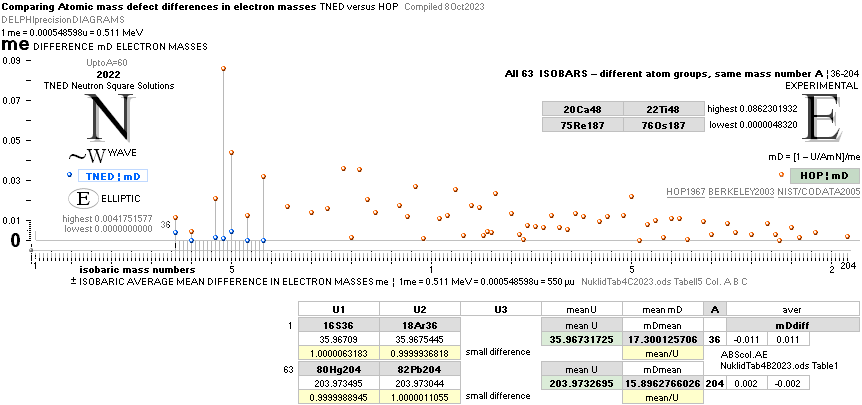
NuklidTab4B2023.ods Table1 Col.Y+
NuklidTab4C2023.ods Table5 Col.A B C
Neutron Square Solutions up to Apr2020 — these isobaric data are the present compiled results on
atoms up to mass number 60 as given from the generalized wave-elliptic Base
Group Mass Numbers equation (2008). NSdeDIA1.
— We still have much work to do
here in UH on completing the basic stable atoms in the nuclide chart on the
Neutron Square solutions. These data now 8Oct2023 are the present available for
comparing purposes.
Based on the
1967 HOP
table atomic masses data:
— Taking the average
mean from any isobaric occurrences over the 63 different atomic families, and
expressing the tabled U value difference to the averaged U in the Neutron
Square atomic mass defect mD electron masses, gives the above diagram: the differences are generally
small.
— The overall
comparing picture exposes differences between Experimental and NeutronSquare
solutions — large enough for a further, deeper investigation.
Shorter: Unless we have missed
some vital parts of the mother ship in coming up on the on ramp, we are just
warming up.
The
isobaric difference and mean average was calculated
NuklidTab4C2023.ods Table5 Col.A B C —
Table1 Col. Y+
isoAVER = (isoU1 + isoU2 + .. + isoUn)/n ¦ nMAX
= 3
mDisoA = (1 — isoAVER/AmN)/me
mDisoDiff = mDisoA — (1 — isoUn/AmN)/me ¦ the
(pair) difference is ± symmetric
See also
further from
TheActualDifferencesHOP/TNED: ExperimentalErrrors ¦ IsobaricMEAN ¦ HIGHESTmD ¦ Comp ¦ Comp2023 ¦ CompareNUCLEAR ¦ CompCALu2023
The Actual Differences TNED/HOP
The TNED atomic nucleus, inner structure N3m20¦15 —
Originally 3D modeled in Simply 3D in Windows 3.1¦95 1994+
THE [‡Table][‡Equation] neutral atom NEUTRON SQUARE ATOMIC MASSES all stable isobaric mean isotopes UP TO A=60
THE DIAGRAMS BUT NOT THE SCALES FROM the free OPEN OFFICE CALCULUS — extremely difficult
and tedious to reach an end result: the OOcalc program has no exact pixel
representation — and changes frequently the precise positional conditions
arbitrarily — however still useful where no other is available. Compare the
more guaranteed exact DELPHIpreciseDiagrams version.
———————————————
u =
1.66033 t25 KG ¦ me = 0.000548598 u ¦ HOP-Berkeley-Nist-Codata ¦ NeutronSquareFundamentals — Geometric
Left: TNED/NS
solutions Difference to HOP — also the established Weizsäcker nuclear mass
defect solution in COMP and ComparingNUCLEAR
¦ ExperimentalErrors.
— The different
TNED/NeutronSquare solution types described in NS solutions ¦ Geometric.
— The above
left chart results came through a (first deduced [‡Original2003][‡Deduction][‡Table], ordered mathematical) MIX between the basic Elliptic-Wave solutions.
— These values
are transferred from the actual original NuklidTab4.wks — after tedious
attempts, Microsoft’s 2008 banning of the MsWORKS: the removal of its cell
coding on the imperative mod operator and the REST command. It was finally (this production) successfully translated to
OpenOfficeCalc cell commands (NuklidTab4A2023TableA) in NuklidTab4B 2023 Table2 Col.J, along with its explaining elliptic-wave mathematics in BaseGroupMassNumbers. How the Code finally after fifteen years came in to
OpenOfficeCalc.
— The wave
parts [‡NKwaveFunction] reflect
generalized values, not elliptic precise. So, there is room for further
precision in the left above chart — however on the more demanding (and time
consuming — yet only manually solvable in this presentation) elliptic equations
credit (EllipticITERATIVEmDSolutions).
NOTE 1: THERE ARE NO STABLE NUCLIDES FOR MASS NUMBERS A=5 AND A=8.
NOTE 2: NS solutions
have only neutral atoms. There are no experimental properties at all — no
experimental charge dependency.
— The experimentally presented stable
nuclides (for starters) have several same mass number A over
several different U atomic families. If this offers a conflict between
experimental and neutral NS solutions — as it would appear — we have at the
present no developed argumentation to settle what is what.
— However again, the differences (IsobaricMEAN)
between these same A but different experimental U [and mD = (1 –
U/AmN)/me] are relatively small. It is at most (all stable
isotopes up to 83Bi209) 0.08623 me = 0.000047305 u = 47.305 µu —
which has no visual representation in the above chart scale. Meaning:
— For comparing NS/TNED with HOP
on the above given scale, it makes no difference if we use the original HOP
table experimental U value — or even »a generalized mean» U value for one and
the same mass number A over the actual occasions.
The
difference as such has — though — other interesting features, worthy of a
separate investigation: Clarifying the significance of the subject — possibly
divulging flaws and errors.
NOTE 3: Also compare on the corresponding established Weizsäcker solution
on NUCLEAR mass defects in FIBAPO: We must exclude (Comp)
the first light atoms from the Weizsäcker results as these are way outside the
vertical scale: any idea of a regular comparison becomes out of the question: the
present academic idea of the atomic nucleus is badly incorrect — in any
precision manner.
— Further Weizsäcker solution
comparisons in Comp, WeizCalifornia,
WeCALu,
Comp2023
and CompCALu2023.
VerticalElliptic: NSsolutions ¦ Geometric
VERTICAL ELLIPTIC
TNEDexperimental ¦ TheActualDifferencesHOP
We are investigating the NS
(Neutron Square) solutions (»carved in the rock») CITER (Carved In ThE Rock) status
against the experimental results — and any academic or other theoretical idea
of solving the mathematics of true atomic masses.
THE AMAZING H2 Be9 O18 EXAMPLE
LBL —
Lawrence Berkeley Laboratory
LBL —
LAWRENCE BERKELEY NATIONAL LABORATORY — Atomic masses, Audi et al., 2003
— was used for comparing basics.
The HOP table 1967 shows similar LBL values
[ later values have more claimed precision ], not really significant in
difference to this present analysis.
ITERATING NEUTRON SQUARE SOLUTIONS BY a simple DETAILED EXAMPLE
——————————————————————————————————————
The iterating aim is to reach a (12 decimal) comparing 1.000000000000 and 0.000000000000
As the effort to
iterate further extends, there is always the possibility that there is a more
precise decimal representation — if the agent has got more time, and can stand
the stress
Having a first clue on a
fusion path (separate analysis) connecting 1H2, 4Be9 and 8O18, A=18
——————————————————————————————————————
On the TNED given/deduced general Neutron Square atomic mass defect (mD) equation
mD = 6 ± √ 602 – (60 – [ A – K]/E)2
we ITERATE (the Iteration Theorem — studying examples) om K and E until we find a closest possible match with a given or proposed experimental value.
The mission is to find — and prove — that the Neutron Square Solutions elliptic atomic mass equations do connect very close paths — close corresponding quantitative physically related values — with the already experimentally observed.
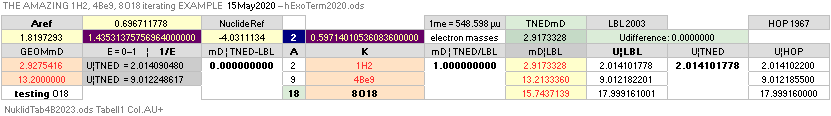
Nuk8O18 — here
rearranged cells for more overview. Original table — CalCard — in hExoTerm2020.ods Table1 B163.
LBL —
LAWRENCE BERKELEY NATIONAL LABORATORY — Atomic masses, Audi et al., 2003
— was used for comparing basics.
The HOP table 1967 shows similar LBL values
[ later values have more claimed precision ], not really significant in
difference to this present analysis.
The above example:
Before a further method was developed on the above (three point iterative) complex, the above result did arrive after an intense three hour expedition: manually flipping, flopping and seeking between the K and E entrance decimals, slowly (the paycheck) observing a growing precision of decimals. The search aim: show us that
T / L = 1.000000000 and T – L = 0.000000000
Or as many decimals we can buy us time for to find — provided undisturbed. Intense interest and concentration is demanded — under absolute natural authority.
— »I am now in the center of
the Galaxy, and I do not accept to be disturbed».
Result:
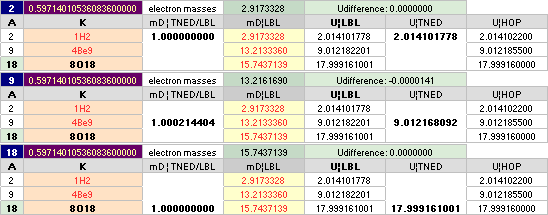
With both 1H2 and 8O18 matching exact the LBL data, a third 4Be9 atomic mass defect shows the T–L U-difference 9.012168092 – 9.012182201 = –0.0000141u = 14.1 µu or -0.02572 me.
— The Neutron Square solution preferences have no ionization connection or other possible impact from other sources. So, the precision should be of type astounding unless we have missed something. It only proves that the Neutron Square solutions mathematics hold the test.
ThePOINT: ».. we are just
warming up ..»
THE POINT — finding the Neutron Square O18 atomic mass solution defect ..
————————————————————————————————
WHEREAS the basic 1H2 and 4Be9 already have defined GroundNuclide atomic mass definitions (About the method ¦ UnequivocalAmD ¦ FIBAPO) — and the elliptic iterative job is to use these for testing experimental results on the higher elements mass defect determination, the next step on the 1H2-4Be9-8O18 expedition was to investigate IF SUCH a defining three point ellipse also exists where — exactly — the differences to 1H2 AND 4Be9 are zero, respectively one in relation. It is exposed in hExoterm2020test.ods Tabell1 Q53:
In this case the 8O18 atom is captured by almost the same ellipse E = 0.696711778, but more precisely an E-vaule of exactly 0.700000000. The mD(O18) = 15.7262112 (LBL15.7437139 and HOP15.743814445), ended on the mD difference of a rounded –0.02 me. About the same as the above 4Be9 difference (–0.026 me).
As a first standard, presenting the general (HOP+) atomic weight/mass table data on a visual computer based pixel precision diagram, we can use the ± 1 pixel tolerance on the 18 me mD scale as a lower least accepted difference:
HOPfirst66: TheEX
mD = (1 – U/AmN)me —
U tabled experimental atomic weights in u = Dalton units = m(6C12)/12,
here: u = 1.660033 t27 KG
—————————————————————————
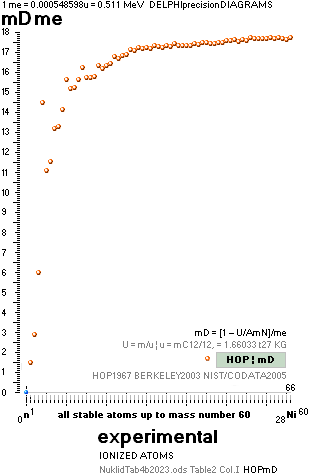
The 66 stable
atoms in the HOP 1967 atomic mass/weight table, as expressed in atomic mass
defect electron masses mD = (1 – U/Amn)me with U = mATOM/u with u = mC12/12 =
1.66033
t27 KG, and A the atom’s mass number, mn and me the neutron and electron mass in u
(Dalton units).
ComparingToleranceStandard: HOPfirst66
If the type TNED–LBL data mD is equal to 0.05 me, a difference of one pixel (standard computer LCD screen) is touched. Below that limit, there is no visual difference between TNED results and HOP/LBL experimental values. The result above on 4Be9 with -0.02572 me lies below that limit. And we would not know, at the present, any method in disregarding such a result as incorrect.
— Accepted first equality limit: ± 1 pixel (±0.05 me: anything below will not be visible in the above scale). So we can use the most exact and sharp visual available tools to present exact (mathematically rounded) data.
On further iterative tests, we would anyway have to set up a form of basic standard, informing us on an iterated result IF it is acceptable as a standard of basic overviewing comparison — or if further precision is needed.
Suggested connection and explanation:
The Neutron Square NEUTRAL Atomic
no experimental encroachment BUILDING HEAVIER FROM LIGHTER
mass defects (mD) versus the experimentally
measured results:
TNED¦NS ← (HOP¦Berkeley¦NistCodata)EXPERIMENTAL ← (WEIZ)AcademicTHEORY
See further resolution from
Short science history
THE NEUTRON
Quotes from a Free PDF @Internet 3Sep2023
THE
FUNDAMENTAL PROPERTIES OF THE NEUTRON ¦ NIST 2009
Fundamental
Neutron Physics Summer School, NIST June 2009
1932:
” Chadwick replaced the paraffin with a variety of other targets and, by measuring the recoil energies of the ejected particles, was able to determine the mass of the neutral particle
Chadwick claimed this was Rutherford’s Neutron ”,
1933:
” Bainbridge makes precision measurements of the atomic masses of the proton and the deuteron using the mass spectrograph ”,
1934:
” Chadwick and Goldhaber make the first ”precision” measurement of the neutron mass by looking at the photo-dissociation of the deuteron
hv + d → p + n
Using 2.62 MeV gammas from Thorium and determining the recoil energy of the protons they were able to determine*:
Mn = 1.0080 ± 0.0005
”,
See also The Neutron Decay as explained by TNED in related physics:
— The Neutron gradually loses
mass rebuildWORKenergy as it decays
(ca 12-14 minutes, np-Structure).
BASICmN: NeutronNIST
Related physics deductions
COMPARING THE NEUTRON SQUARE INTERNAL APPROXIMATED
NEUTRON MASS VALUE: 1.008644262901 .. u
U = (mN/2mDme)(1 — √ 1 —
1/[mN/4mDme]) ; as deduced in Neutron’s
AtomicWeight Value
U = (mN/2[15.6]me)(1 — √ 1 —
1/[mN/4(15.6)me]) ;
U = ([mN/me]/2[15.6])(1 — √ 1 —
1/[(mN/me)/4(15.6)]) ;
U = ([mN/me]/2[15.6])(1 — √ 1 —
4(15.6)/[(mN/me)]) ; mN/me
minimum = 1836 :
1818 + 18
———————— = 606 + 6 = 3 × (»±101»
+ »±1») ;
Discovery — nuclear structure, the neutron
3
U = (1836/2[15.6])(1 — √ 1 —
4[15.6]/1836) ;
U = (918/15.6)(1 — √ 1 —
15.6/459) ;
U = 1.008644263 ;
mN approximated
Ume = mN/1836minimum = 0.0005493705 ; me approximated
HOP
source: mN
= 1.0086652 ¦ me = 0.000548598 ¦
mN/me = 1838.6235458387
Neutron’s
AtomicWeight Value
— the first minimum approximation
mNminimum
= (1836/[2·15.6=31.2])(1 — √ 1 — 1/[1836/[4·15.6=62.4]]) = (1836/31.2)(1
— √ 1 — 1/[1836/62.4]) = 1.008644262901 ..
That is one decimal position better
than the above quoted 1934 James Chadwick measured approximation 1.008.
— However note:
This TNED/NS deduction, the equation (11), builds upon — is totally promoted by
— already existent known experimental data (1900+). What we know (at the
present) it cannot be inferred outside those premises — unless some (ancient,
long forgotten) genius FIRST discovered the Neutron Square
Fundamentals »as carved in the rock» — and from there made
further conclusions, theoretical and/or practical. In any way, that is not our
present era of the related scientific way.
(Children have a [strong natural] tendency
of exposing such breakthroughs .. anyway ..).

NuklidTab4C2023.ods Tabell1 A20
Explain:
The NS solutions mD value in electron masses (me) for
mass number A=12 is exactly 15.6 — mass numbers 5
and 8 have no stable atomic nuclear isotopes.
As observed (The atomic mass unit
— u):
— The natural choice of an atomic mass
unit
(u,
Dalton, here 1.66033 t27
KG = m[6C12]/12)
ends (1960+) on the stable carbon atom
6C12.
— In our science history one first adopted 1H1 for u, then 8O16, and finally 6C12 [1960+].
— A correlated corresponding real
experimental physics u-value would then match the equation 11 above (The
Neutron’s Atomic Weight Value).
PRIME: BASICmN
Taking the TNED (mathematical)
structural provisions
(prime number base: the nuclear stability
ends on not whole number divisible quantities)
based on the general top quantum number
3 (TNED’s first N3m20
— HOW), there is apparently not much to chose on:
— With a ±e nuclear (from The Neutron) structure —
with a possible Electric
Displacement (defining
a net nuclear charge [Z] and magnetic moment) — the choice, as based on
the neutron atom (enclosing the hydrogen atom), is
1818 central
massif ¦ Central Contacts
18 expendable for mass defects mD in building heavier
from lighter — Nuclide AZ map
These whole numbers (connecting The
Periodic System by whole number
solutions: the Nuclear
matrix algorithm: Kepler resonances)
ends on the prime numbers: 1 3 101. That certainly locks up a certificate for
the atomic/nuclear stability, as noted
1818 + 18
———————— = 606 + 6 = 3 × (»±101»
+ »±1»)
3
So the theoretical end station — after
(Nov2007) some in between reckoning (Explaining The atomic mass unit — the atomic, not
nuclear, mass defect principle) — ended on the deduced equation (11)
— giving us directly a first simple approximation of the neutron atomic
weight/mass value mN = 1.0086..
u.
Planck constant h = mcr = The Neutron =
mN × c0 × rN —
See The Proton Radius in related physics
ATOMIC MASS — AND THE NEUTRON SQUARE
As hinted at above (NeutronMass):
— Atomic mass, beginning from the
Neutron — concealing the Hydrogen atom — can in no way be measured
experimentally without affecting the subject of measure by changing its neutral
unaffected conditions.
While the precision of the measuring
method, technically, can be extended to »unlimited numbers of decimals», the
practical part of the quest — the actually unaffected mass — needs additional
calculating work — uncertainties over the time of change — before any end value
arrives on the table.
Meaning:
The experimental way on atomic mass
determination has inside of itself no precise or exact parametric preference.
The resulting values can and will differ, more or less, depending on
experimental method — and its time consuming length: the amount of affection.
GETTING TO THE POINT:
So:
— How do we know
that the NS Neutron Square solution connects to
any experimental results at all?
First Bold:
— By proving it
is supreme to any theoretical mathematical attempt on explaining a
corresponding true atomic result.
Because if it isn’t: — what is the use of it? Answer.
Apparently none. Completely worthless.
Answer:
— We know so by comparing (THE
ATOMIC NUCLEUS) — inspecting by the eye on any resolvable scale
— the neutral atom’s Neutron Square internal solutions — the values — with the
experimental and theorecial whatever results on these two atomic mass
defect transfer expressions:
mD =
(1 — U/AmN)/me :
U =
AmN(1 — mDme)
————————————————————————————————————————————
mD atomic
mass defect i electron masses me ¦ ClarifyingTheUequation
¦ AtomicMassDefectEquation
the actual
wasted mass-energy difference in building a heavier atom K from lighter K1 + K2:
— these mD values are calculated in the Neutron Square
solutions, then compared with [ freely available ] experimentally tabled values
on atomic weight values U: The NS solution has internal mD:s, resulting in
calculable U:s, then comparable to the experimentally available tabled U values
from different laboratories.
me electron
mass 1me = 0.000548598u
= 0.511 MeV
mN neutron
mass 1mN = 1.0086652u
U =
m(ATOM)/u
u =
m(6C12)/12
=
1 Dalton unit (u) [”Da”]
=
1.66033
t27 KG
A mass
number
the number of original neutrons making up the actual atom —
in related physics through exothermal nuclear fusion
reactions only:
— in modern corridors: A = n + p = A—Z + Z, Z atomic number or nuclear-electronic
charge
TNEDexperimental: NSdeDIA1: EnhancedPrecision
———————————————
The
Generalized WaveElliptic Equation ¦ NeutronSquareSolutions
E experimental
U-values transfered to mD values by mD = (1 — U/AmN)/me
generally based
on instrumentally activating changes in the atomic neutral and unaffected
condition
N the
TNED Neutron Square internal solutions — here by The generalized
WaveElliptic formula
GENERAL VERTICAL ELLIPTIC
and WAVE-elliptic — The ELLIPTIC EQUATION
¦ GEOMETRIC
¦ WaveMETHOD
entirely based
on geometric/paragonic structure provisions, based on Planck constant
h=mcr=Neutron.
See TheAtomicNucleus,
The ProtonRADIUS,
The PlanckRING
unless already familiar.
The different methods here are overviewed in NeutronSquareFundamentals.
By the same mD equation, the NS values
can transfer on U values, and thereby directly to atomic weights/masses, U =
m(ATOM)/u = AmN(1 — mDme).
The fact that, the left diagram above,
the experimental (E) over the neutron square values (N) differ (1pixel = 0.05
me = ————) only marginally proves beyond any doubt in the very first place:
the NS
solution complex apparently has a real steel solid not possible to destroy
natural nuclear physical connectivity. If the reader can disclaim: please do
share.
In the second place — if the reader has
no objections — the coherence proves that nature — not human academy — rules
science:
apparently and
so provably by internal (eternal) indestructible paragon structures — »carved
in the rock» — like a flower in the field
(mathematical matrices: nuclear
structure, the periodic system: all basic mathematical concepts: Mathematics5:
what we humans find if willing to dig).
More Weizsäcker comparing
details will follow on the (13Oct2023) in a way very new and different
California University Weizsäcker recently found Weizsäcker equation. See from WeizCalifornia.
It has (in most parts) superseded the below comparing results.
In the third place — the academic way
of consenting (beginning from around 1900), not deducing, ideas on atomic and
nuclear physics has a comparable diagram to the above: the Weizsäcker equation
solutions (WeizsäckerDetails):
WAcademicExperimental:
NSdeDIA2:
B(Z,N ¦ MeV) = 14A – 0.61Z(Z–1)A–1/3 – 14A2/3 – 84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 ¦ HOP1967 NSdeDIA10
The WEIZSÄCKER SOLUTION ON ITS FIRST 1967¦1975
TESTED FORM
———————————————
WeizsäckerDetails ¦ HOPweizQuote1967
NuklidTab4B2023.ods Tabell3 — WEIZSmD Col.L
HOPweizXP.ods Tabell1 — has the table
construct [ somewhat extensive ] from which the Weizsäcker values have been
extracted as above.
E experimental
U-values transfered to mD values by mD = (1 — U/AmN)/me
generally based
on instrumentally activating changes in the atomic neutral and unaffected
condition
W the
Weizsäcker nuclear mass defect solution.
B(Z,N ¦ MeV) = 14A
– 0.61Z(Z–1)A–1/3 – 14A2/3 –
84.2(A/2 – Z)2/A ±¦0: 34.0A–3/4 ¦ HOP1967 NSdeDIA10
See HOPweizQuote1967 and WeizsäckerDetails.
See also the original comparing
Experimental/TNED/Weizsäcker diagram in
ComparingTNED/MAC
(UH Nov2007): TNEDcomparingMAC.
The first two Weizsäcker atoms lie
outside the vertical scale.
See also the diagrams on the actual
differences between the combatants in
Comp and ComparingNUCLEAR.
— While the TNED/NS
solution (TEX) exposes an apparent minimum of
differences, the academic theoretical solution exposes: not really a succesful
idea of the atom and its nucleus. No way. Not even close.
TAP0solutions2020:
EnhancedPrecision:
The latest 2020 results from enhancing the General
wave solution by Geometric and
precise Elliptic iterations — as of 6Oct2023
PRECISION ELLIPTIC OVER WAVE GENERAL
TNED History
Up to May2020 (TNEDatomPhysics0 — TAP0)
the NS precision elliptic solutions (Geometric
¦ NSsolutions
¦ EllipticVertical)
only had a provision of the light atomic chart up to mass number 60.
Enhancing the (VerticalElliptic)
elliptic method by introducing a first manual iteration procedure (FEfullTNED ¦ TheEXAMPLE
¦ The Iteration Theorem)
the generalized regular wave-elliptic
solution could be superseeded by a more precise actual atomic mass number
Neutron Square regular elliptic (»exact») solution (CheckTabAElliptic — Extracted examples).
It should be
noted here (4Sep2023) that no further Neutron Square Solutions
work has been done since May2020 on examining the entire stable atomic chart up
to (at first) mass number 60. The iterated elliptic equation results presented
here only rely on those past represented results in TAP0.
(And, at present; working with the solutions by a manual iteration Example
on each atom is, still, a demanding and time consuming enterprise).
The most NS solution favorable
compared to the experimental
——————————————————————————————————————————
In selecting
the more exact solutions — exposing a possible closer match
(NuklidTab4C2023.ods
Tabell2 Col.G i L V) — the following (6Oct2023) compiled picture holds:
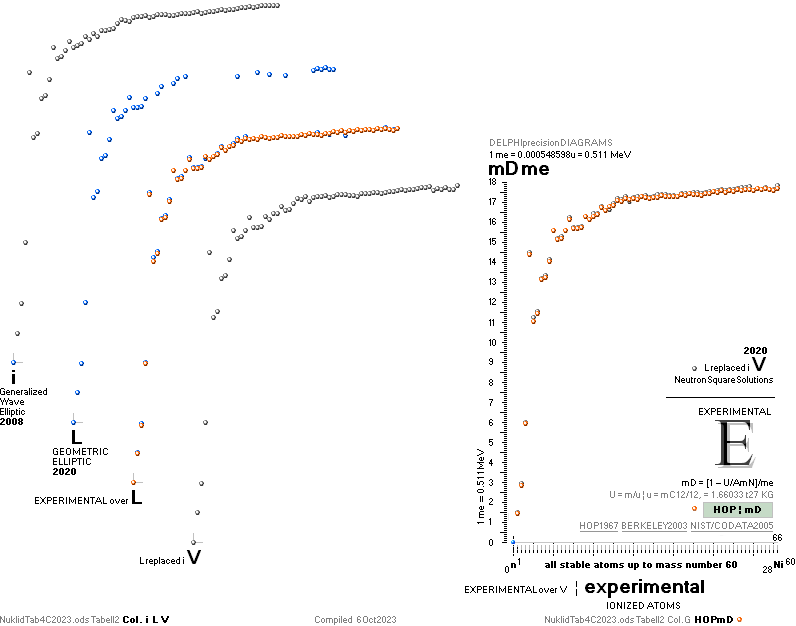
NuklidTab4C2023.ods Tabell2 Col. i ¦ L ¦ V ¦
G
———————————————
The marginal differences NSdeDIA1 between Experimental and NS
solutions just underlines the essential:
— Unless a deeper natural
connection does exist in atomic and nuclear physics between actual
experimentally collected values and the static geometric paragonic NS results,
the pictured coherence would — by logic — never have shown. And now that it
does show: It is apparently systematically of the type: tight. Real steel
stuff, »carved in the rock» so to speak.
We can use the Carved In The Rock specification (NS CITER) for a further test
on its credibility in comparing all available data on the atomic and nuclear
masses complex.
The above presented, derived from
TNED¦N NSdeDIA1,
is just a first preliminary overviewing picture on the results from May2020 (TNEDa0).
We are (here in UH) far from finished on these deeper (hopefully more precise)
elliptic iterative solutions.
As these are of the more demanding and
time consuming nature, time is not really on the side of a single individual to
carry out a more extensive investigation — unless healthy enough to carry on
for some further 514 years, give or take ±12.
The present available results are
sufficient enough to stress the central point:
•
the coherences between TNED and Experimental have an apparent provable
common nature;
•
the small differences are small enough to urge a deeper inspection of
the experimental nature (laboratory details,
not normally exposed freely @Internet) and its parametric composition,
comparing on the NS solution results.
The end picture above to the right shows
that the TNED deduced nuclear and atomic complex definitely connects to the
experimental complex. However on smaller differences here not yet clarified.
Taking the NSdeDIA3 TNED/Experimentally values on
differences in atomic weight values (U) and atomic mass defect (mD) on the end
Experimental over V diagram renders the following view :
ATOMIC WEIGHT AND ATOMIC MASS
NuklidTab4C2023.ods Tabell2 Col. Z ¦ AA
Apparently the first basic Question:
WHAT IS THE REASON FOR THE DIFFERENCES
— especially the increasing aspect, growing from
lighter to heavier:
♦ The
NS
solution is apparently a static/geometric/VerticalElliptic
—
not much we can do about it, what we know, especially so in concern of
the first five posts: 0n1, 1H1, 1H2, 2H3 and 2He4
— while the experimental is a dynamic: entailing
many (complicated) parametric/instrumental involved parties on the most
technically sophisticated developed level (1932+).
We have no (explicit) answer to that
question here (yet).
(We refuse to speculate here: proof is
needed).
Especially because of the tight first
light elements — greater difference on heavier — there is a growing urgent
reason to investigate a rational explanation:
The NS solutions
have only neutral atoms — no charge dependency
•
The NS solutions describe neutral atoms: no affection: should
be »linear».
•
The Experimental results uses ionizing (hf-quanta), plus relativistic
ideas on conversion between mass and energy (E=hf=mcr/t=mc²), introducing a
complex of other conditions than the neutral aspects.
Exactly which is this further aim to penetrate.
— Compare: AllKeplerMath
— present scientific academic community
appears to be in deep cosmological theoretical trouble (the death of modern
academic ideation).
See also SPECIFIC NOTES
— details on the above
complex-comparing NSdeDIA3.
SPECIFIC NOTE TO THE NSdeDIA3 COMPARING DIAGRAM
NSsolutionsTNEDmD 1H1: 1.512616535
The Neutron Square’s special transfer
expression
— rP/rN = (√8)/(1 + √3) =
1.0352761804 involving the two basic atomic and nuclear agents: the neutron and
the proton radius
— offers an option of averaging.
———————————————
The ProtonRADIUS ¦ Geometric
Given the exact geometric (Geometric)
NS solution to the 1H1 atomic mass defect mD(1H1) = 1.461075377, there is the
option
mD(1H1) = mD(1H1GEO) × rP/rN
=
1.512616535 ;
HOPmD =
1.518021871 ;
mDdiff =
-0.0054 ..
Compared to the pure geometric mD
difference (-0.0569), the rP/rN option in absolute is more than a ten times
precision improvement.
It has been used in the NSdeDIA3
enhanced comparing diagram in the replacing L Geometric-Elliptic.
CLAIR: 17Oct2023 — The WikipediaWeizsäckerQuote ¦ ClarifyingTheU
THERE IS NO ESTABLISHED ARTICLE IN THE SCIENTIFIC COMMUNITY ON ATOMIC MASS DEFECT
The different concepts of atomic and nuclear mass defect — exemplified
CLARIFYING EXAMPLES IN PRESENT LITERATURE
ATOMIC AND NUCLEAR
There are some (several) flourishing misconceptions and delusions — (almost) impossible to resolve with the present established set of scientific community vocabulary — (as proven for starters by this example, Oct2023):
” The page ”Atomic mass defect” does not exist”.
Wikipedia 9Oct2023.
A search on ”mass defect” on the other hand is redirected in
Wikipedia to the article on
Nuclear binding energy.
nuclear mass defect
atomic mass defect MDa = AmN – U ¦ U = mATOM/u ¦ u = m(6C12)/6 = 1 Dalton
nuclear mass defect MDn = AmN – U – Zv ¦ v = nino = mN – (mP + me) = mN – m1H1 (= 0.00084u)
MDn – Zv = MDa MDn = MDa + Zv.
v: the work mass energy waste that built the
first atom — 1H1 Hydrogen — from the fundamental atom: The Neutron [ FAMQ
]
WEB@INTERNET EXAMPLE:
Confusions arise on the idea that ”mass defect” is a unitive concept. It is not.
It is necessary to specify weather ”mass defect” relates to atomic or nuclear.
— But that was also a hell of
a way to put it, in light of the above sampled:
— The term for clarification is not even represented.
The Internet example
(many domains have this
typical formulation, none mentioned):
”
A mass defect is the difference between an atom’s mass and the sum of
the masses of its protons, neutrons and
electrons.”
@INTERNET search 10Oct2023
As interpreted on atomic mass defect:
MDa = ZmP + (A–Z)mN + Zme – U
= ZmP + AmN – ZmN + Zme – U
= AmN + Z[mP – mN] + Zme – U
= AmN – Z[mN – mP] + Zme – U
= AmN – Z[mN – (mH1 – me)] + Zme – U
= AmN – Z[mN – mH1 + me] + Zme – U
= AmN – Z[v + me] + Zme – U
= AmN – Z[v + me – me] – U
=
AmN – Zv –
U ¦ the NUCLEAR MASS DEFECT
quantity
related definition. Not the atomic.
Unless so clarified, grave misunderstanding may arise —
»impossible to sort out: .. I hate you ..».
No.
MDa = AmN – U.
As interpreted on nuclear mass defect:
Yes.
MDn = AmN – Zv – U
We take the quote on the word by an example and see how it works:
HOP table Example on Iron ¦ ZATOMAmD ¦ U
——————————————————
26Fe5617.759142302 ¦ 55.9349363 u :
——————————————————
mP 1.00727660200000u
mN 1.00866520000000u
me 0.00054859800000u
——————————————————
NUCLEAR MASS DEFECT:
protons + neutrons +
electrons – atom’s actually (measured) mass ¦ per number A of neutrons
mN that built the atom
26mP + (56–26)mN +
26me – 55.9349363 ¦ ÷ AmN ¦ ÷ me mass defect
expressed in units of electron masses
= 0.5284749 ÷ 56mN = 0.009355980 ¦ ÷ me = 17.0543464337
——————————————————
ATOMIC MASS DEFECT:
56mN – 55.9349363 = AmN – U ¦ ÷ Amn / me
= 0.5503149 ÷ 56mN = 0.009742630 ¦ ÷ me = 17.7591423022
See Wikipedia quote in Not
represented.
See further
The mass defect concept
DIFFERENCE BETWEEN ATOMIC AND NUCLEAR.
See also similar comparing (confusing unless related)
WikiWEIZ:
WikiWeisNUCm
Checking on Academically established atomic and nuclear — exemplified and related WikiWEIZnuclearMass
THERE IS NO ESTABLISHED ARTICLE IN THE SCIENTIFIC COMMUNITY ON ATOMIC MASS DEFECT
THE WIKIPEDIA WEIZSÄCKER QUOTE ON THE BINDING ENERGY
CONCEPT AND EXPRESSION
See Wikipedia quote in Not
represented.
As quoted,
” The semi-empirical mass formula therefore provides a good fit to heavier nuclei, and a poor fit to very light nuclei, especially 4He.”,
WIKIPEDIA,
Semi-empirical mass formula (as quoted 15Oct2023).
The Wikipedia article leaves no comparing diagrams or other comparing quantitative results on the differences between calculated and experimentally measured. Here we (only until recently) have the HOP 1967 source (and the Swedish FOCUS MATERIEN 1975) concurrent values.
Wikipedia on the Weizsäcker
nuclear binding energy equation
—————————————————————————
WIKIPEDIA, Semi-empirical mass formula (14Oct2023)
” It was first formulated in 1935 by German physicist Carl Friedrich von Weizsäcker,[2] and although refinements have been made to the coefficients over the years, the structure of the formula remains the same today.”,
” The formula gives a good approximaltion for atomic masses and thereby other effects. However, it fails to explain the existence of lines of greater binding energy at certain numbers of protons and neutrons. These numbers, known as magic numbers, are the foundation of the nuclear shell model.”.,
” The formula represents the liquid-drop model proposed by George Gamow,[1] ..”,
WikiWEIZnuclearMass: WikiWEIZquote ¦ RelatedNUCLEAR
” The formula The mass of an atomic nucleus, for N neutrons, Z protons, and therefore
A = N + Z nucleons, is given by
m = Zmp + Nmn – EB(N,Z)/c2
where mp and mn are the rest mass of a proton and a neutron respectively, and EB is the binding energy of the nucleus.”,
WIKIPEDIA, Semi-empirical mass formula (as quoted 15Oct2023).
Clarifying the rank
INTERPRETING THE WikipediaQUOTE — here, our UH designations unless misunderstood (no direct quote exists, so there is a certain margin of hazard here):
m = Zmp + Nmn – EB(N,Z)/c2
”The mass of an atomic nucleus”:
Mnuc = ZmP + (A–Z)mN – B ¦ ”The mass of an atomic nucleus”; B = EB(N,Z)/c2 ;
= ZmP + AmN – ZmN – B
= AmN + ZmP – ZmN – B
= AmN + Z(mP – mN) – B
= AmN – Z(mN – mP) – B ¦ mN = mP + me + Zv
gravitating proton mass + gravitating electron mass
= AmN – Z(me + v)
– B ¦ m, the
Wikipedia proposed nuclear
mass
B, ”the binding energy of the nucleus” ;
AmN – B =
Mnuc+ Z(me+v)
AmN = Mnuc+ Z(me+v) + B
------------------------------------------------
Checking — related — the
Wikipedia rank
—————————————————
Checking — related — the Wikipedia rank
—————————————————
Mnuc = U – Zme ¦ nucleus gravitating mass + electrons gravitating mass = atom gravitating mass:
: Wiki:
AmN – B = Mnuc+ Z(me+v)
AmN = Mnuc+ Zme + Zv + B ;
AmN = U – Zme+ Zme + Zv + B ;
AmN = U + Zv + B
B = AmN – U – Zv
B ”the binding energy of the nucleus”
; verified
nuclear mass
defect — related
physics
MDn = AmN – U – Zv
AmN – U – Zv = nuclear mass defect ¦ MDn
AmN – U = atomic mass defect ¦ MDa = total atomic binding energy =
absolute max binding energy
Summing
The Wikipedia RANKING Quote (certainly) certifies that the underlying implied conceptual idea behind the Weizsäcker equation on ”binding energy” connects to the NUCLEAR — not atomic — mass defect concept.
That is comforting — comparing the (erlier 1967¦1975) different Weizsäcker equation results.
Health and care issues on
planet Earth — outside modern academic quarters
WHY PLANTS¦LIFE PREFER O16 BEFORE O18
Connecting the DOLE1965
observations
————————————————
Oct2023 — a final (strong) possible
proof and resolution (quoted) on the respiratory disease
phosphorylation oxygen (O18¦16)
molecular connection
to deforestation, as suggested by several earlier articles[‡]
in the UniverseHistory productions: the general global biochemical connected
deforestation complex (investigating basic
life matrix chemistry).
————————————————
———————————————
Related physics and
mathematics
——————————————
In CWON from CAP
there is already an atomic/nuclear proven
connection to a preorganic »chemical matrix» system. It has a provided proof on
already known samples from meteorites NOT TO SAY how THE ALREADY EXISTING EARTH
LIFE BIOLOGY here came on (Primary Neutron Nuclear Carbon Matrices):
principally and deeply verified.
All (CAP) primary surface celestial
bodies develop an organic surface nuclear-atomic matrix structure from the
K-cell expansion, and further.
The reason, as noted, lies or can be related
so to lie in the concept of STRUCTURE — beginning from the fundamental atom:
the neutron: Planck constant h=mcr. Normally within 12-14 minutes, a regular
stable Hydrogen atom appears out of the unstable neutron atom/nucleus.
FastForward: Each celestial body develops its own specific surface structural
combinations, depending on the body’s mass (Surface synthesis — CWON).
Phosphorylation: PO4
So the central Quest in this article
was: The PO4 molecule:
phosphorylation
a Phosphorus
atom15P31 no
isotope with 4 Oxygen atoms O16 isotopes O17¦18 hooks up — or triggers other events —
with a larger biochemical structure
@INTERNET
¦ WIKIPEDIA Phosphorylation (28Sep2023)
” In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology.”.
Cell division, mitosis PO4 agent:
The PO4 component is, as testified in
many texts, the (finally 1970-1980) discovered agent — biochemical mechanics —
responsible for most (not to say all) biological events connected to cell
division (mitosis): DNA-RNA, general amino acid and (glucose) protein structure
input-output organization and maintenance. The »Commander DoIt».
— What about PO4
— what are you trying to say?
Specifically unknown in modern
corridors:
The PO4 CAP atomic/nuclear primary
chemical matrix — proven in CWON — only allows or at a first
glance appears so to allow a regular (biochemical) matrix with
O16. That is: In precise concurring observation with DOLE1965 (deforestation
already during 150 years beginning from 1800, see MLN):
(»PO4-mechanics»)
organic plants »prefer» O16 over O18.
At first, as noted: There is an eleven
percent gain in mass/energy: 1—16/18=0.11 ..: Using O16 is the most effective
and biological economic.: fastest, least losses.
At second, the real steel argument
— however not apparent in modern
corridors, the CWON (proof) from CAP complex, responsible for organic structure
— is this:
As preliminary tested: P with O18
offers no direct primary regular biochemical structure
(here known — no direct proof: we are talking origin of Earth life
here). It is readily obvious NOT appropriate for a primary natural
repetitive chemical molecular chain construct (with smallest amount of blanks:
the effective covering primary volume structure, exemplified and illustrated
below).
The hint is:
PO4 with O18 is
(definitely) not suited for life biochemistry. No way. O16 it is.
StrongHINTEDproof:
Phosphorylation
———————————————
The O16 is the (CWON) natural component in the biological
CO2 cycles. Deforestation breaks that state, dumping normally ground based
cyclic natural O16 into excessive atmospheric CO2 — where the nearest ground (air gases after atomic weight ¦ OxygenFilter: CO2 the heaviest followed by) replacement
is: O18. Result: impoverishment of the natural life sustaining biological
health foundation. That was the original reflection here in UH (BioEK1 ¦ Present state atmospheric lies
way below: 1254
¦ TheTEN):
Deforestation kills — suffocates — life. A statement to be biochemically
proven. Now the PO4 suggests a serious link to such a (final) proof: life power
decrease. Pandemic situations. Affecting both plants and animals.
No direct obvious primary
atomic/nuclear chemical matrix for 1(P) + 4(O18).
— Possibly PO2. No more.
So, IF O18 associates chemically with P, no
natural such biochemistry ever existed ..
— says — or strongly suggests — CWON from CAP.
1(P) + 4(O16) on the other hand, seems
to reflect excellent biochemical primary matrix systematics — as too the other
exemplified parts (amino acids, chlorophyll and hematin exemplified in KemBlockEX).
The
DOLE1965
preferred O16
There is an eleven percent gain in
kinetic (mv) mass/energy: 1—16/18=0.11 ..: Using O16 is the most effective and
biological economic.: fastest, least losses.
is
primarily not because of the 11% mechanic-electric argument.
The
primary reason (CWON
from CAP)
is: THAT IS HOW LIFE HAS BEEN ASSEMBLED. During its rough 3 billion years on
earth, organic life began by an already inherited atomic/nuclear ordered
STRUCTURE (periodic system) — all from the birth of the Earth as a celestial
body (VoJ).
TNED and its related physics strongly suggests so — based on atomic/nuclear
structure. Not random and chance. No pharmaceutical industry. No unintelligent
Mother Nature. No bald cuts.
— These arguments and aspects lie totally outside
the horizons of present academic cosmological ideas. No way. Intelligence is
under question.
HintedConsequences:
HntedProof
So (SAN1), on assumed relevant proceedings:
What happens IF natural biology is
FORCED to replace O16 with O18 — because a huge amount of the original natural
cyclic O16-C12 has been dumped in the atmosphere because of industrial
exploration on alive forests, not allowing them to live and die naturally (the root system guaranteeing biodiversity,
Simard2012). A created growing amount of bald cuts on Earth compromises life
sustainability:
— What happens to Life biochemistry?
———————————————
SDandCWON ¦ MLF ¦ MaunaLoaProof ¦ MLN1812IPCC2013Graph284ppmvCO2 ¦ WHAT PUSHES THE TREND
RISE?
BioEK1 (heavily compromised by deforestation
1812+) with the
naturally normal forest preference MLN1812IPCC2023Graph284ppmvCO2 as proven in CWON guarantees the primary difference between biological CO2
cyclic O16 and general atmospheric O16 — these have (VoJ) a well related separate geological history explanation
(not in present established corridors) — and do not mix on credit of a
preserved natural biology. A definite (heavy, natural, scientific) dispute is
so lying on our table.
Shorter — on the presumed relevant
quest:
What happens (Phosphorylation) with a Life,
educated during some 3Gy on (15P31)(8O16)4
if it suddenly is forced by human PhD
interests from some 1812+ and further pharmaceutical industry 1850+, now during
a rough 150y, to develop on a »life alien species» of the form
(15P31)(8O18)4 ?
.. slower .. demanding more effort ..
problematic connection ..
QUOTES: Con ¦ Phosphorylation
Quotes ...
”
All organisms produce a phosphate compound, ATP, which is the universal
energy currency of life.”,
WIKIPEDIA , Photophosphorylation
(27Sep2023)
:
”
During respiration and photosynthesis
Phosphorylation is essential to the processes of both anaerobic and
aerobic respiration, which involve the production of adenosine
triphosphate (ATP), the "high-energy" exchange medium in the cell. During aerobic respiration, ATP is synthesized in
the mitochondrion by addition of a third phosphate group to adenosine
diphosphate (ADP) in a process referred to as oxidative phosphorylation.
ATP is also synthesized by substrate-level phosphorylation during glycolysis.
ATP is synthesized at the expense of solar energy by photophosphorylation in
the chloroplasts of plant cells.",
WIKIPEDIA ,
Phosphorylation (27Sep2023)
:
”
The phosphorylation serves two purposes:
to activate each already-assembled pre-replication complex, and to prevent new complexes from forming. This
ensures that every portion of the cell's genome will be replicated once and only once. The reason for prevention of
gaps in replication is fairly clear, because daughter cells that are missing
all or part of crucial genes will die. However, for reasons related to gene
copy number effects, possession of extra copies of certain genes is also
deleterious to the daughter cells.”,
WIKIPEDIA, Cell cycle
(27Sep2023)
:
”
Protein phosphorylation is an important
cellular regulatory mechanism as many enzymes and receptors are
activated/deactivated by phosphorylation and dephosphorylation events,
by means of kinases and phosphatases. In particular, the protein kinases are
responsible for cellular transduction signaling and their hyperactivity,
malfunction or overexpression can be found in several diseases, mostly
tumors.”,
”
This reversible mechanism occurs
through protein kinases and consists of the addition of a phosphate group (PO4)
to the polar group R of various amino acids. Consequently, this addition modifies the protein from hydrophobic apolar to
hydrophilic polar, allowing
the protein to change conformation when interacting with other molecules.”,
THE CRUCIAL ROLE OF PROTEIN PHOSPHORYLATION IN CELL
SIGNALING AND ITS USE AS TARGETED THERAPY
Ardito et al., Published online 2017 Jun 22 (25Sep2023)
INTERNET SEARCH
»Phosphorylation and Covid» 28Sep2023:
phosphorylation
shown in coronavirus
” Glycogen Synthase
Kinase-3 Regulates the Phosphorylation of Severe ...
https://www.ncbi.nlm.nih.gov › pmc ›
articles › PMC8011290
The involvement of GSK-3 in N phosphorylation has also been shown in another
coronavirus, JHMV. Finally, we found that inhibition of GSK-3 could
suppress the replication of both coronaviruses. The results not only indicate
that GSK-3 is critical for N phosphorylation but also suggest its involvement
in regulating viral replication.
”.
coronavirus
proteins predicted be phosphorylated
” Role of phosphorylation
clusters in the biology of the coronavirus ...
https://www.ncbi.nlm.nih.gov › pmc ›
articles › PMC7103301
Although coronavirus N proteins were
predicted to be phosphorylated at multiple serine residues, mass spectroscopic
analyses identified two regions of phosphorylation on the IBV N protein (when
over-expressed) which are located at two conserved amino acid clusters, Ser 190
and Ser 192 and Thr 378 and Ser 379 (Chen et al., 2005).
”.
phosphorylation
sites
conserved in
coronavirus proteins
” The Global Phosphorylation
Landscape of SARS-CoV-2 Infection
https://www.sciencedirect.com › science
› article › pii › S0092867420308114
The cluster of phosphorylation sites
within an arginine/serine (RS)-dipeptide rich region, C-terminal to the RNA
binding region (Figure 2 A), is conserved in other coronavirus N proteins. This
region is phosphorylated in SARS-CoV by serine-arginine (SR) protein kinases,
modulating the role of SARS-CoV N protein in host translation inhibition ( Peng
et al., 2008 ).
”.
Eighty-seven compounds
identified
global
phosphorylation profiles
representing
potential COVID-19 therapies
” The Global
Phosphorylation Landscape of SARS-CoV-2 Infection
https://pubmed.ncbi.nlm.nih.gov ›
32645325
Eighty-seven drugs and compounds were identified
by mapping global phosphorylation profiles to dysregulated kinases and
pathways. We found pharmacologic inhibition of the p38, CK2, CDK, AXL, and
PIKFYVE kinases to possess antiviral efficacy, representing potential COVID-19
therapies.
”.
systematic study
of phosphorylation
and
phosphoproteins help find
suitable
therapeutics of COVID-19
” Frontiers | SARS-CoV-2
Infection Triggers Phosphorylation: Potential ...
https://www.frontiersin.org › articles
› 10.3389 › fimmu.2022.829474 › full
17 feb. 2022A systematic study of the
dynamic change in phosphorylation site and phosphoproteins will help to find
suitable therapeutics of COVID-19, especially suitable kinase inhibitors.
SARS-CoV-2 affects various kinases including CMGC, CK2, CDK, and PKC, and also
regulates important signaling pathways including MAPK cascade, GFR signaling
...
”.
phosphorylation
of STAT1 enhanced
in severe
COVID-19 cases
” Altered increase in STAT1
expression and phosphorylation in severe COVID-19
https://pubmed.ncbi.nlm.nih.gov ›
34676541
Contrary to the baseline STAT1
expression, the phosphorylation of STAT1 was enhanced in severe COVID-19 cases,
indicating a dysbalanced JAK/STAT signaling that fails to induce transcription
of interferon stimulated response elements (ISRE). This abnormality persisted
after IFN-α and IFN-γ stimulation of PBMCs from patients with severe
COVID-19.
”.
current study
aimed understanding
SARS-CoV-2
protein
influenced by
phosphorylation
” Phosphorylation of
SARS-CoV-2 N protein affects its function
https://www.news-medical.net › news ›
20200701 › Phosphorylation-of-SARS-CoV-2-N-protein-affects-its-function.aspx
The current study is aimed at
understanding how the N protein is influenced by phosphorylation.
Characterization of N protein condensates. an SDS-PAGE analysis of all N
protein mutants used in...
”.
Phosphorylation
of disordered region
underlying
mechanism is not known
” Phosphoregulation of
Phase Separation by the SARS-CoV-2 N ... - PubMed
https://pubmed.ncbi.nlm.nih.gov ›
33248025
Phosphorylation of the central
disordered region promotes the protein's transcriptional function, but the
underlying mechanism is not known. Here, we show that the N protein of
SARS-CoV-2, together with viral RNA, forms biomolecular condensates.
”.
modulation of phosphorylation
” Nucleocapsid mutations in
SARS-CoV-2 augment replication and ... - PLOS
https://journals.plos.org ›
plospathogens › article?id=10.1371 › journal.ppat.1010627
21 juni 2022Our results indicate that
changes in N phosphorylation correlate with differences in virus replication;
thus, we sought to modulate N phosphorylation using kinase inhibitors. Prior
work has identified two consensus sites for GSK-3 phosphorylation within the SR
domain and inhibition of GSK-3 has been shown to reduce SARS-CoV-2 replication
( Fig ...
”.
And many others.
SUMMING Internet articles on PO4 versus Covid:
The advertising headlines — having identified the phosphorylation PO4 as an active agent — reflect an underlying pharmaceutical effort to control its PO4 agency of biochemical mechanics — by pharmaceutically testing disease inhibiting molecular compositions at least not directly (½-2 year tests) having any negative effects on the patient.
SHORTER — unless disclaimed
Present pharmaceutical industry is playing with fire. No doubt.
Modern academic ideas of highly manipulative medicine have a max 70 years experience (1950+), compared with Mother Nature’s roughly 3Gy — on a PO4 assembling life maintaining credit (still 2 eyes, not 3).
No way.
Other Quotes:
” Early cell cycle studies established that phosphorylation was important for cell division. ",
p2Col1t —
DISSECTING THE MECHANISMS
OF CELL DIVISION, Ong et al.,
JBC Papers in Press. Published on June
7, 2019 ¦ Free PDF (28Sep2023)
:
”
This is because protein phosphorylation
has gradually become an integral part of all the systems they are studying
themselves. Indeed it would be difficult to find anyone today who
would disagree with the statement that
“the reversible phosphorylation of proteins regulates nearly every aspect of
cell life.”,
”
Phosphorylation and dephosphorylation,
catalysed by protein kinases and protein
phosphatases, can modify
the function of a protein in almost every conceivable way;
for
example by increasing or decreasing its
biological activity, by stabilizing it or marking it
for destruction, by facilitating or
inhibiting movement between subcellular compartments, or by initiating or
disrupting protein–protein interactions.”,
”
The simplicity, flexibility and reversibility of phosphorylation,
coupled with the ready availability of
ATP as a phosphoryl donor, explains its selection as the most general
regulatory device adopted by eukaryotic cells.”,
THE ORIGINS OF PROTEIN
PHOSPHORYLATION, Philip Cohen , 2002
Free PDF (28Sep2023) — [eukarotic-,
which have a nucleus]
IF NOT ALREADY PART OF A FUNDAMENTAL PROOF ..
These quotes, at
this stage, does not prove anything else than a strong hint. The needed proof
is: A thorough — mass — spectroscopic analysis of the (PO4 associated
biochemical) molecular content. At first clarifying IF the (general) O in the
PO4 is of type O16 — or what is feared here: of type O18.
[ beginning at 1812: dumping the recycling natural CO2
by deforestation, creating a corresponding biochemical biological disturbance,
gradually evolving through many forms of disease ]
— Possibly: We
are just looking at the top of the iceberg. Deforestation continues.
While State
administration should alert a global alarm, nothing of the kind is seen.
Perhaps such mass spectroscopic O16-O18
analysis already has been made. But none of it is seen at the free non-cookies
established Internet now September 2023.
Many (especially medical) sites are —
also — blocked to the visitor, demanding a prioritizing cookies consent for
site access — blocking, interrupting, the actual serious scientific interest.
It reflects treating the visitor as an infant, really, in deep need of support
for further life from a more acquainted instance than the individual scientific
interest : no access.
Nature, and other medical
associated:
Declare it,
then: »This
website is NOT for any average human to access on serious scientific interests».
— »The Visitor will be interrupted, asked
to bow, and consent, before other inducements».
— Not one word HumanRight recognition. Not a sound. Not a spell. Not a hint.
”.. every individual and every organ in society ..”, ”.. of the greatest importance ..”. No sound.
Wickedness — evil — continues to beat up Violence, intrusion, rape .. spanking .. domination ..
— Again: by DRIFT. Not plan. With zero teaching and education on basic care and responsibility — UDHR10Dec1948: no sound — humanity is caught in a daily expanding trap of impossible intercoursing solutions other than .. violence, despair, desperation .. explored by the police, prosecutor, court — and the screaming public — to practice punishment and obedience. No sound. Not one word. Evil — no care — knocks on Violence’s door. No moral.
Short:
Around 1800 a type enters Europe forcing the populations to cooperate under laws of violence by threat of punishment.
The authoritative pressure has since forced the populations to adopt with solidarity and loyalty: obedience and punishment on a general scale of most popular conduct: spanking. Rape.
— So: When did that cease?
No teaching. No education.
Just obedience and punishment. A deep yearn for domination.
— »Here, you do as you are told. And that is the end of it».
NuclearReactionLaw: Exothermal nuclear reaction law — SWEDISH EDITION, full deduction and
explanation¦ NUCLEAR REACTION LAW — connecting expression
to general cosmic state law — related energy equivalents
NUCLEAR REACTION LAW
———————————————
Exothermal nuclear reaction law ¦ NUCLEAR REACTION LAW ¦ FusionRINGS
GIVEN THE RELATED DEDUCED EXPRESSION, the nuclear reaction law uses already established tables of atomic masses/weights for evaluation — and determination of the basic involved parameters. The nuclear reaction law mathematics tests IF two (or three) given atomic nuclei can form a heavier atom and its nucleus by exothermally emitting Planck energy (m→γ) E=hf taken from the involved fusing masses [K1 + K2 – (m→γ) = K].
Example (hExoterm2020.ods Tabell1 — autoFormat):
NOTE: the free OpenOffice spread sheet cell program here has been
ADOPTED FOR USE WITH LAWRENCE
BERKELEY NATIONAL LABORATORY ATOMIC MASS TABLE 2003
LBL —
LAWRENCE BERKELEY NATIONAL LABORATORY — Atomic masses, Audi et al., 2003
NOT ENOUGH fusion mass to generate exothermal energy:
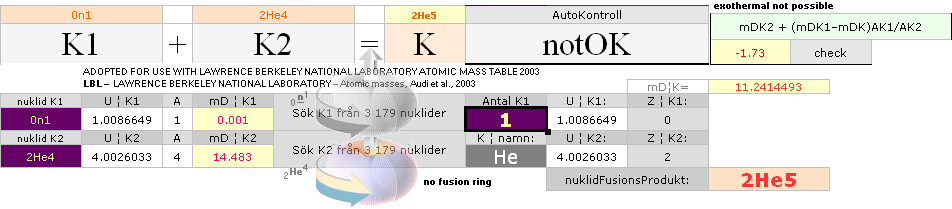
SUFFICIENT fusion mass to
generate exothermal energy:
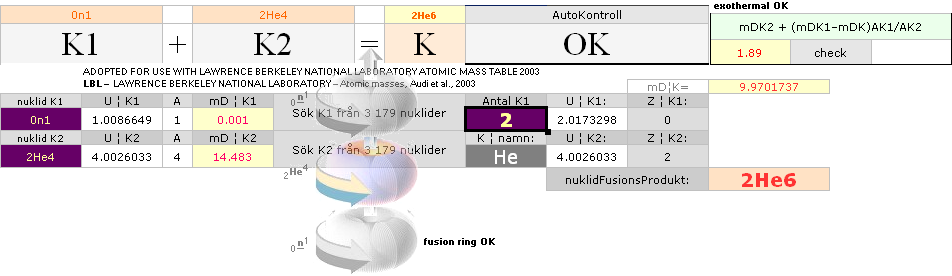
The result generates the LBL table
parameters — and the corresponding Neutron Square values (the actual view of
parameters here are reduced for clarity).
Based on the results, further analysis
continues on NeutronSquareSolutions.
NOTE: In modern
corridors — no TNED theory — the above may very well
exist by mathematical principle. But given the basics of the TNED complex, it
is not likely that we ever will see any of the above kind in type Wikipedia or
other established encyclopedia. Wikipedia is reserved for the modern academy
history alone: ”related sources — not truth”. Safely separated.
NScredit: 20Oct2023 — LGDbasics ¦ NSUnit
Weizsäcker solution is chasing the experimental
and the experimental is chasing the neutral — the NS
neutral atomic masses:
the experimental
cannot dispense with electric and magnetic field strengths:
The Neutron Square solutions has nothing of the kind.
WHAT IS THE ENIGMATIC AND STRANGE STATUS OF THIS
REMARKABLE NEUTRON SQUARE SOLUTION PARAGONIC
»CARVED IN THE ROCK» EDIFICE? AND HOW DID IT COME HERE? AND WHY WAS IT NOT
DISCOVERED BEFORE?
Short review:
THE ORDERS AFTER WHICH THE NEUTRON SQUARE WAS DISCOVERED AND
REVEALED ¦ Discovery
Planck constant the universal efficacy quantum (Max Planck 1900) ¦ TheNEUTRON
h = mcr
= 6.62559 t34 JS
= mN · c0 · rN ¦ mass charge spin ANGULAR MOMENTUM — mvr, Kepler momentum vr
= 1.0086652u · 2.99792458 T8 M/S · rN ¦ u = 1.66033 t27 KG = m(6C12)/12
rN = h/mNc0 ¦ neutron gravity circle radius ¦ the fundamental PlanckRING radius
= 1.319897164124040 t15 M
NUCLEAR STRUCTURE mass charge spin: electric displacement
±e — secured indivisible on a specific basic prime number:
1818 ÷ 18 =101 cannot be further divided — »carved in the universal rock»:
(1818+18)/3 = 606 + 6 = 3 × (»±101» + »±1») › See HOW3.
Total number of electron-positron masses in the neutron: 1818+18+k: (k=2.624..¦ mN/me=1836 + 2.623545838670)
1818 central
massif
18 maximum (never
touched) mass-energy waste per neutron in building heavier atoms
26Fe56 HighestmD
17.7591423022 electron masses [ the discovery 2003 ]
NS the
neutron square
with square side mass number 60 ¦ Elliptic Equation
rP ProtonRADIUS
r0 = 1.37 Fermi
— Planck constant on Hydrogen spectrum’s
energy circle:
The Neutron Square solution outclasses/challenges present scientific community idea on the nature of nuclear physics
EXPERIMENTALLY MEASURED ATOMIC WEIGHTS/MASSES VERIFIES NEUTRON SQUARE SOLUTIONS
as so observed:
— »carved in the universal
rock»:
— GRAVITATION — the neutron atom, the fundamental form of gravitational mass, the Planck Ring — is not a particle.
— CHARGE (Z) is not mass, does not interact with mass, although integrated with mass in the atom.
— Gravitation is not light (charge: electric and magnetic properties).
— Light is not gravitation.
— Light is not a particle.
— Curved light paths does not develop centrifugation: light is massless.
— Max Planck was right. Albert Einstein was wrong.
..
normalized
on u = m(6C12)/12: U = 12 = m/u
TNED ← EXPE ← WEIZ
NORMALIZED
Weizsäcker solution is chasing the experimental
and the experimental is chasing the neutral — the NS
neutral atomic masses:
the experimental
cannot dispense with electric and magnetic field strengths:
The Neutron Square solutions has nothing of the kind.
StandardUniversal: Discovery
PO4 ¦
ATOMIC MASS defect
EQUATION ¦
ComparingNUCLEAR
¦NScredit
EXPERIMENTALconfirmations: Atomic
masses
THE MACHINE THAT RUNS THE WHOLE SHOW:
Light’s Top Divergence c0 is preserved independent of gravitation’s influence on light’s propagation in space:
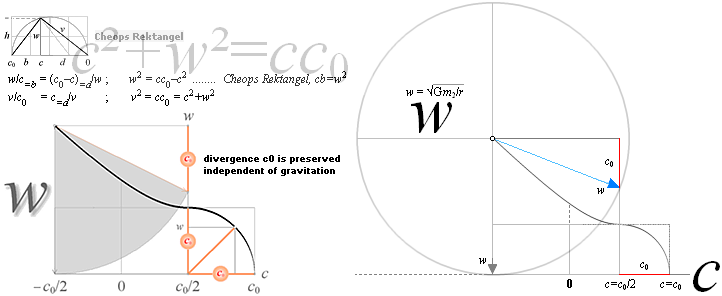
HRB w2 – c2 = c02 ¦ CRL w2 + c2 = c02 ¦
c = (c0/2)(1 ± √| 1 – (2w/c0)2 |) light’s gravitational dependency LGD ¦ w = √Gm2/r = c ¦ 2c2 = cc0 ¦ c = c0/2 = cz energyZone: ¦ c0 = 2√G[m2/(n→∞)]/[r/(n→∞)]:
c0 is preserved independent of any possible
[m/(n→)]/[r/(n→∞)] fractal nuclear construct: the fundamental
atom, the neutron,
the fundamental form of gravitation: gravitation is not a
particle. No way.:
local
gravitational dominance has the strongest stand in the atom, the atomic
nucleus, the neutron — its Planck fractal hollow ring toroid structure,
not its volume
G = (c0/2)2(r/m2)
= c02(r/4m2) = c02([r/(n→∞)]/4[m2/(n→∞)])
; c0 = √4G[m2/(n→∞)])/[r/(n→∞)]
— it is dynamic, not static. It comes with the ±e structure of the atom.
NO BEGINNING— it was never created ¦ NEVER CREATED, NEVER DESTROYED — c0 ¦
Relating mass — physical existence — to atoms, the fundamental neutron, and the neutron to a fundamental (Planck Ring hollow TNED toroidal fractal) ±e nuclear structure (EXPERIMENTAL CONFIRMATIONS), the (Newton) universal law of gravitation exposes a forcing rank expression leading to the deduction of Light’s Gravitational Dependency (Fig.1: Related and comparing · Einstein · Schwarzschild). Its related and comparing mathematical connection to the fundamental neutron (The origin of energy = mass ¦ EnergyLaw) becomes as stated above. This Universe History (UH) knows of no other sources (EXCON).
THE ONLY KNOWN UNIVERSALLY DIRECT EXPLAINABLE PROOF
SHOWING THAT THE NATURE OF DIVERGENCE (light: electric, magnetic and thermal) c
HAS A TOP HIGHEST VALUE c0 =
2.99792458 T8 M/S AbsoluteMetrics INDEPENDENT OF THE INFLUENCE OF GRAVITATION
— while at the same time the actual propagation of light in space IS highly determined by the
presence of gravitational potential (GPS-example ¦ Swedish Original GPS) IS: Light’s
macroscopic cosmic universal gravitational dependency LGD.
Divergence c0 is preserved independent
of gravitation’s influence on light’s propagation
in space
See also (Sw. version original) Negative divergence.
BackGround — all the necessary
introductory concepts
The microscopic
gravitation property — the atom, the atomic nucleus (the fundamental neutron) —
has not the macroscopic gravitation property. These apparently reflect two
different realms of the aspect and idea of reality: gravitation is not a
particle. No way.
AS YET (Gtest) FOUND and related ABSOLUTE NATURAL CONSTANTS (in UH universe history):
c0 2.99 792 458 T8 M/S
light’s divergence ¦ AbsoluteMetrics ¦ G, universal gravitational
constant:
G fundamental but not absolute determined — tested on the IAU PSUN in TNED ¦ 6.670 t11 JS/(KG)2
h mN c0 rN, not absolute
determined ¦ 6.62559 t34 JS Planck constant
— the neutron ¦ mass ±e-charge spin
e fundamental but not absolute determined ¦ 1.602 t19
C electron’s electric charge
u m(6C12)/12, not absolute determined ¦ 1.66033 t27
KG universal atomic mass unit
rN fundamental but not absolute determined ¦ 1.32
Fermi neutron
g-circle spin radius
rP = r0 = (me/mN)(1/π√8 + π[1–me/mN])h/mec0π
= (rN = h/mNc0) × (√8)/(1 + √3) ¦ 1.37
Fermi proton
g-circle spin radius
mN fundamental but not absolute determined ¦1.0086652u neutron mass
me fundamental but not absolute determined ¦ 0.000548598u
electron
mass
ε0 1/(4π t7 AM/VS · c02)
= 8.8541878176 t12 C/VM ¦ IAUtest electric
constant
PSUN IAU value, 3.8275 T26 W ± 0.0014, TNED tested
in K-cell heat physics on all the above Sun’s
photometric effect
mEARTH 5.975 T24 KG (CWON from CAP)
Earth’s
mass —
suggested a natural constant
m0cK K-CELL MASS at the moment of its detonation 4.161432507433896 T53 KG
r0c K-CELL RADIUS at the moment of its contraction 1.544177741 T26 M
CalCards: Kalkylkort: NOTE. OpenOffice SpreadSheet. Swedish
EditionOnly
PART OF THE AIM behind these open
(author’s working original) available CalCards is of course
•
first the availability of the proving mathematics on exact basics
•
with the possibility for any interested reader to make own tests, or
further, whatever
•
offering a complete open access index to the complete work behind the main text :
•
we leave no one behind in related physics and mathematics, as far as we
can.
HOPweizXP.ods ¦
HOPweizXPa.ods
¦ NuklidTab4A2023.ods ¦ NuklidTab4A2023.ods ¦ NuklidTab4A2023.ods
——————————————————————————————————————
1967HOP atomic mass table —
HANDBOOK OF PHYSICS, E. U. Condon, McGraw-Hill 1967 ¦ Atomviktstabellen i HOP
allmän referens i denna presentation, Table 2.1 MASS TABLE ¦
s9–65—9–86
——————————————————————————————————————
THE SWEDISH OPEN OFFICE CELL CODED
VERSION —
we do not know how the cell code looks if opened in an English version
— if at all ;
— We should have thought about that from the start [ 2008 ]
— which we didn’t.
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN HOPweizXP.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/HOPweizXP.ods
T1
The 1967HOP¦1975FM
here in UH first observed Weizsäcker equation solution ”nuclear binding energy”
parametric matrix and values
T2
The 2008 original UH collected CODATA/NIST values up to 83Bi209, all stable
atoms
T3
The corresponding 1967HOP original data
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN HOPweizXPa.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/HOPweizXPa.ods
T1
The markedly enhanced (13Oct2023) found California university New
Weizsäcker
equation — with drafts and results as in the main text
T2-T3
same as in the above XP.ods
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN NuklidTab4A2023.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/NuklidTab4A2023.ods
TA
BaseGroupMassNumbers General elliptic wave atomic mass
defect Neutron Square solutions up to mass number 60
TB
1967HOP
table index for comparing
T1
constants
T2
1967HOP data up to 103Lw257 ¦ results and drafts to TA
T3
the original (2008) MsWORKS NuklidTab4.wks constants — used for the final
OpenOffice transitions
T4
The70 isotope resulting/proving cell
coding A-Z chart from TA
T5
General drafts and results up to 83Bi209.
NuklidTab4B2023.ods — Contined
and enhanced works from NuklidTab4A2023.ods
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN NuklidTab4B2023.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/NuklidTab4B2023.ods
T1
constants with drafts and results as specified in the main text
T2
1967HOP
table data for further drafts with results as specified in the main text
T2A
1967HOP table data for further drafts and results as specified in the main text
T3-T5
1967HOP table data for further drafts and results as specified in the main text
TA
the original 1967HOP table data — manually transferred (2000) from the library
book
TB
all stable 1967HOP table isobaric
NuklidTab4C2023.ods — THE
MOST/latest UPDATED AND COLLECTED DATA from the above AB spouses
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN NuklidTab4C2023.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/NuklidTab4C2023.ods
T1
constants — with drafts and results as specified in the main text
T2
1967HOP
table data — with drafts and further results as specified in the main text
T2A
1967HOP table data — with drafts and further results as specified in the main
text
T3-T4
1967HOP table data — with drafts and further results as specified in the main
text
T5
isobaric 1967HOP table data ¦ the GPS table comparison on two different c-preferences
T2A
The 1967HOP table data with further drafts and results
TB
comparing HIGHESTmD
ALL
AS LISTED: HOPweizXP.ods ¦
HOPweizXPa.ods
¦ NuklidTab4A2023.ods ¦ NuklidTab4A2023.ods ¦ NuklidTab4A2023.ods
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN HOPweizXP.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/HOPweizXP.ods
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN HOPweizXPa.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/HOPweizXPa.ods
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN NuklidTab4A2023.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/NuklidTab4A2023.ods
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN NuklidTab4B2023.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/NuklidTab4B2023.ods
kalkylkorten nedan DIREKT FRÅN DEN
HÄR WEBBLÄSAREN NuklidTab4C2023.ods
— se öppningsmanual
om ej redan bekant — eller kopiera URL:en nedan till valfri webbläsare
(vilket som fungerar — förutsatt att SVENSKA VERSIONEN av gratisprogramvaran
OPEN OFFICE finns installerad på datorn)
http://www.universumshistoria.se/AaKort/NuklidTab4C2023.ods
Atomic Nucleus II 2023VII15
The Atomic
Nucleus
innehåll — content
2008VIII6 ¦ 2023IX10
The Atomic Nucleus II — NeutronSquareMATH 2003-2023
[HOP]. HANDBOOK OF PHYSICS, E. U. Condon, McGraw-Hill
1967
Atomviktstabellen i HOP allmän referens i denna presentation, Table 2.1 MASS TABLE ¦ s9–65—9–86 ¦
concurrent — with such minor end decimal differences with Berkeley National 2003 and Nist/Codata 2005 — having no significance in this presentation
Comparing CODATA2005-HOP1967 ¦
———————————————————————————————————————————————————————
mn =
1.0086652u
...................... neutronmassan i atomära massenheter (u)
[HOP Table 2.1 s9–65]
me =
0.000548598u .................. elektronmassan
i atomära massenheter (u) [HOP Table 10.3
s7–155 för me , Table 1.4 s7–27 för u]
m(1H1) = 1.007825200u .................... neutronmassan i atomära massenheter (u) [HOP Table 2.1 s9–65]
u = 1.66043 t27 KG .............. atomära massenheten [HOP Table 1.4 s7–27,
1967]
u = 1.66033
t27 KG .............. atomära massenheten [ENCARTA 99 Molecular
Weight]
u = 1.66041 t27 KG
............... atomära massenheten
[FOCUS MATERIEN 1975 s124sp1mn]
u = 1.66053886 t27 KG ........ atomära
massenheten [teknisk kalkylator, lista med konstanter SHARP EL-506W
(2005)]
u = 1.6605402 t27 KG .......... atomära massenheten [@INTERNET (2007) sv. Wikipedia]
u =
1.66053906660
t27 KG .... atomära massenheten [@INTERNET (2023) en. Wikipedia, Atomic mass]
u = 1.660538782 t27
KG ...... atomära massenheten [från www.sizes.com],
CODATA
rekommendation från 2006 med toleransen ±0,000 000 083 t27 KG (Committe
on Data for Science and Technology)]
c0 = 2.99792458 T8 M/S ........ ljushastigheten
i vakuum [ENCARTA 99 Light, Velocity, (uppmättes i början på
1970-talet)]
h = 6.62559 t34 JS ................. Plancks konstant [HOP s7–155]
e = 1.602 · t19 C .................... FOCUS MATERIEN 1975s666
—
Det internationella standardverket om universum sammanställt vid universitetet
i Cambridge, The Cambridge Encyclopaedia of Astronomy, London 1977.
[FM]. FOCUS MATERIEN 1975 — Fysikens, kemins och astronomins
historia. Allt från atomen till universum — fysik, kemi, jordvetenskap och
astronomi
[BKL]. BONNIERS KONVERSATIONS LEXIKON, 12
band A(1922)-Ö(1928) med SUPPLEMENT A-Ö(1929)
t för 10–, T för 10+,
förenklade exponentbeteckningar
t för 10–, T för 10+, förenklade exponentbeteckningar
PREFIXEN FÖR bråkdelar och potenser av FYSIKALISKA STORHETER
Här används genomgående och konsekvent beteckningarna
förkortning för förenklad potensbeteckning
d deci t1
c centi t2
m milli t3
µ mikro t6
n nano t9
p pico t12
f femto t15
Alla Enheter anges här i MKSA-systemet (M meter, KG kilo[gram], S sekund, A ampere), alla med stor bokstav, liksom följande successiva tusenprefix:
K kilo T3
M mega T6
G giga T9
T tera T12
Exempel: Medan många skriver cm för centimeter skrivs här konsekvent cM (centiMeter).
MAC, här ofta använd förkortning för Modern
ACademy — etablerad vetenskap
sedan början av 1800-talet
In UH often used abbreviation for modern academy — explicitly from the beginning of the 1800s
MAC — often used abbreviation in TNED for Modern ACademy
TNED — Related PHYSICS And MATHEMATICS —
Se särskild djupbeskrivning av innebörden i begreppet relaterad framställning.
Toroid Nukleära
Elektro MEKANISKA Dynamiken
(Toroid Nuclear Electromechanical Dynamics), eller Toroidnukleära Elektromekaniska
Dynamiken är den dynamiskt ekvivalenta resultatbeskrivning som
följer av härledningarna i Planckringen h=mnc0rn,
analogt Atomkärnans Härledning.
Beskrivningen enligt TNED är relaterad,
vilket innebär: alla, samtliga, detaljer gör anspråk på att vara fullständigt
logiskt förklarbara och begripliga, eller så inte alls. Med TNED förstås
(således) också
RELATERAD FYSIK OCH MATEMATIK. Se även uppkomsten av termen TNED
i Atomkärnans Härledning.
SHORT ENGLISH —
TNED in general is not found @INTERNET except under this domain
(Universe[s]History, introduced @INTERNET 2008VII3).
TNED or Toroid
Nuclear Electromechanical Dynamics is the dynamically equivalent resulting
description following the deductions in THE PLANCK RING, analogous AtomNucleus’
Deduction. The description according to TNED is related,
meaning: all, each, details claim to be fully logically explainable and understandable,
or not at all. With TNED is (hence) also understood RELATED PHYSICS AND
MATHEMATICS. See also the emergence of the term TNED
in AtomNucleus’ Deduction.
Senast uppdaterade version: 2025-03-16.
*END.
Stavningskontrollerat-SpellChecked up to: .. 10Sep2023 ¦
27Oct2023.
rester
*
åter till portalsidan · portalsidan
är www.UniversumsHistoria.se
∫ ∫ Δ √ Δ ≠ → ∞
γ √ ω π τ ε ħ UNICODE — ofta använda tecken
i matematiska-tekniska-naturvetenskapliga beskrivningar
— Ctrl+Shift+Q i Microsoft WORD direkt till SYMBOL
σ
ρ ν ν υ π τ γ λ η ≠
√ ħ ω →∞ →γ ≡ ¦ Alt+ 1..9 ☺☻♥☺♦♣♠•◘○
υ Ψ
Ω
Φ Ψ Σ Π Ξ Λ Θ Δ
α
β γ δ ε λ θ κ π ρ τ φ
ϕ σ ω ϖ ∏ √ ∑ ∂ ∆ ∫
≤ ≈ ≥ ˂ ˃ ← ↑ → ∞ ↓
ϑ
ζ γ λ ξ
Pilsymboler, direkt via tangentbordet:
Alt+24
↑; Alt+25 ↓; Alt+26 →; Alt+27 ←; Alt+22 ▬
Alt+23
↨ — även Alt+18 ↕; Alt+29 ↔
Senast uppdaterade version: 16 mars
2025 | 05:46:44 | 2025-03-16. [GMT+1]Solar [GMT+2]Industry
*
BILDKÄLLA: Författarens arkiv
· 3Jul2012 E12 Bild 198;293 · Nikon D90 ·
Fria Teckningar — Angående Statens och Kommunernas
uppmärksammade UPPENBART SJUKA NATURUPPFATTNING — civilisationens
återerövring.
Denna webbsidas ansvariga författare kan nås med e-mail på
Webbadressen
¦belldharma¦SNABELA¦universumshistoria¦PUNKT¦se¦ MEN SKICKA INTE MED LÄNKAR
— utom överenskommelse kasseras sådan e-post omgående
åter till portalsidan · portalsidan är www.UniversumsHistoria.se